
Is Inorganic Glass
An Inorganic Polymer?
Glass at the Molecular Level
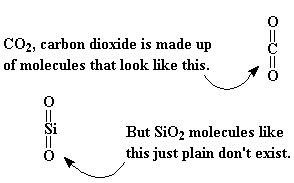
We talk about glass from time to time when we're discussing polymers, especially when we're talking about composite materials. Glass fibers are often used to reinforce polymers. But what is this stuff called glass? We use it with polymers a lot, obviously, but is glass itself a polymer?Before we tackle that question, let's take a look at what glass is. The highest quality glass has the chemical formula SiO2. But this is misleading. That formula conjures up ideas of little silicon dioxide molecules, analogous to carbon dioxide molecules. But little silicon dioxide molecules don't exist.
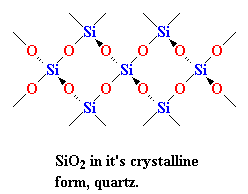 Instead, in nature SiO2 is often found as a crystalline solid, with a structure like you see on your right. Every silicon atom is bonded four oxygen atoms, tetrahedrally, of course; and every oxygen atom is bonded to two silicon atoms. When SiO2 is in this crystalline form we call it silica. You've seen silica before. When you find big honkin' crystals of it we call it quartz. When we have a lot of little tiny crystals of it, we call it sand.
Instead, in nature SiO2 is often found as a crystalline solid, with a structure like you see on your right. Every silicon atom is bonded four oxygen atoms, tetrahedrally, of course; and every oxygen atom is bonded to two silicon atoms. When SiO2 is in this crystalline form we call it silica. You've seen silica before. When you find big honkin' crystals of it we call it quartz. When we have a lot of little tiny crystals of it, we call it sand.
But this silica isn't glass. We have to do something to it first to make it into glass. We have to heat it up until it melts, and then cool it down really fast. When it melts, the silicon and oxygen atoms break out of their crystal structure. If we cooled it down slowly, the atoms would slowly line back up into their crystalline arrangement as they slowed down. (Remember, heat is really just the random motion of atoms and molecules. Hot atoms move a lot, cold atoms move very little.)
But if we cool it down fast enough, the atoms of the silica will be stopped in their tracks, so to speak. They won't have time to line up, and they'll be stuck in any old arrangement. They'll look something like this: v
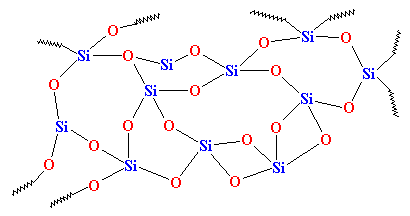
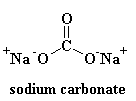
As you can see, there is no order to the arrangement of the atoms. We call materials like this amorphous. This is the glass that is used for telescope lenses and such things. It has very good optical properties, but it's brittle. For everyday uses, we need something tougher. Most glass is made from sand, and when we melt down the sand, we usually add some sodium carbonate. This gives us a tougher glass with a structure that looks like this:
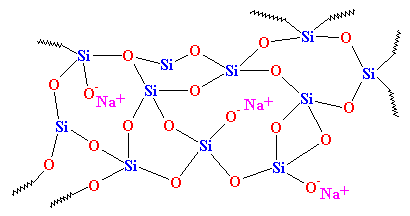
This is the glass you see everyday, in jars and windows, and it's the glass that's used in composites. In fact, it used to be called "soda glass" to distinguish it from quartz.
So is this a polymer or not? Usually it isn't considered as such. Why? Some may say it's inorganic, and polymers are usually organic. But there are many inorganic polymers out there. For example, what about polysiloxanes? These linear, and yes, inorganic materials have a structure very similar to glass, and they're considered polymers. Take a look at a polysiloxane:
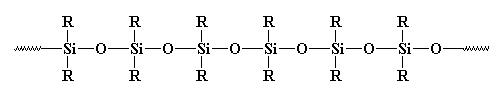
So What About Crosslinking?
In one sense, glass could be considered a highly crosslinked polysiloxane. But we usually don't think of it that way. Why not? Probably because even in a highly crosslinked system, you could still trace a polymer chain and see where the crosslinks are. But with glass, it'd be tough to do that.Here's a comparison that illustrates this point: carbon fibers and carbon nanotubes. Throw in some diamond to make this family complete. "What family?" you ask. Simply the one of carbon bonded to other carbons in a two- or three-dimensional array, and NOT bonded to anything else. Pure carbon!
Why Isn't Completely Crosslinked Carbon a Polymer?
Now remember that carbon really wants to be bonded to four other atoms. Usually these are four distinct other atoms, but multiple bonds to the same atom count as well. That's where most vinyl monomers come in: they have a carbon-carbon double bond, which means that each carbon in the vinyl can only be bonded to two other atoms besides the carbon at the other end of the double bond: four bonds total.Now for the interesting part. In graphene, carbon fibers and carbon nanotubes, the carbons are ONLY bonded to other carbon atoms. In these compounds, however, each carbon has a combination of double and single bonds, making for a highly delocalized set of molecular orbits. That means that these materials conduct electrons and they're electrically conductive. Interesting experiment: put a bunch of carbon fibers in a microwave and zap it: flame and smoke as the electrons moving around in the material react with oxygen and go up in smoke!
Diamond is even more interesting, being the hardest material known. Why is it so hard, yet transparent and able to refract light so beautifully? It's just pure carbon, after all, but with a catch: now each carbon has four single bonds to four other carbon atoms. This perfectly symmetrical array of carbons has a perfect array of bonds going in four different directions. "It don't get no better than this," you might say.
So are these all-carbon networks thought to be polymers? Nope, and for the same reason that glass isn't. It all comes down to convention and history. Ever since we figured out what an organic polymer actually looked like, glass and diamond just didn't fit in. Bias, if you will, since they do meet the requirement of a completely crosslinked polymer. Oh, well, other battles to fight...

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |