
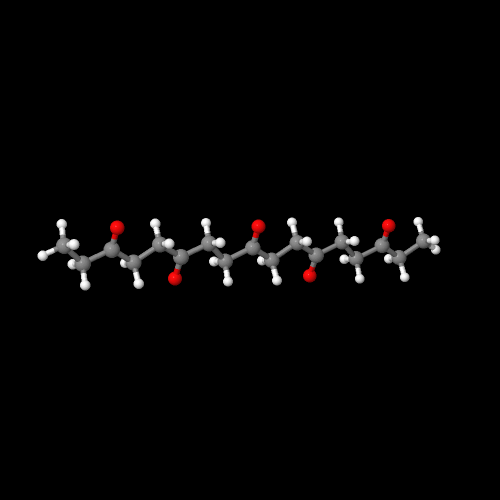
The model on the right above is an image of the pdb model
you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
I would just love to tell you that many wonderful things are made of aliphatic polyketones, but I can't. You see, these polymers are so new that not much has been made out of them yet. But polyketones, which are plastics, by the way, have a lot going for them, and we figure it won't be long before a lot of stuff is made from them.
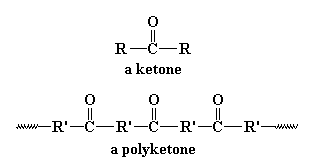 So what's so great about polyketones? Let's find out by taking a look at
them. A polyketone is of course a polymer with a ketone group in
the backbone chain. The polyketones we're going to talk about on this
page are based on the picture you see at the top of the page, where
R' is an ethylene linkage, -CH2CH2-. Shell
has just put this family of polymers on the market and sells them under
the name Carilon. This is not to be confused with a carillon,
which is a musical instrument with differently pitched bells, which
are often
controlled with a piano-like keyboard. Let's see what one of these polymers looks like, next to a chain of good
ol' polyethylene for comparison:
So what's so great about polyketones? Let's find out by taking a look at
them. A polyketone is of course a polymer with a ketone group in
the backbone chain. The polyketones we're going to talk about on this
page are based on the picture you see at the top of the page, where
R' is an ethylene linkage, -CH2CH2-. Shell
has just put this family of polymers on the market and sells them under
the name Carilon. This is not to be confused with a carillon,
which is a musical instrument with differently pitched bells, which
are often
controlled with a piano-like keyboard. Let's see what one of these polymers looks like, next to a chain of good
ol' polyethylene for comparison:
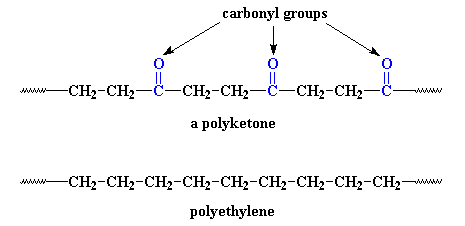
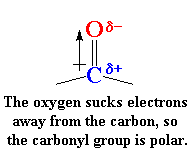 It's not much different. The only difference is that the polyketone has
those carbonyl groups in it. But those carbonyls do a lot. You see,
carbonyl groups are very polar.
This is because oxygen is electronegative
and draws electrons away from the carbon atom. So oxygen has a slight
negative charge and the carbon has a slight positive charge.
These polar carbonyl groups are attracted to each other, and very strongly
at that. This attraction is so strong that while polyethylene melts at a
mere 140oC, the polyketone doesn't melt until 255 oC!
It's not much different. The only difference is that the polyketone has
those carbonyl groups in it. But those carbonyls do a lot. You see,
carbonyl groups are very polar.
This is because oxygen is electronegative
and draws electrons away from the carbon atom. So oxygen has a slight
negative charge and the carbon has a slight positive charge.
These polar carbonyl groups are attracted to each other, and very strongly
at that. This attraction is so strong that while polyethylene melts at a
mere 140oC, the polyketone doesn't melt until 255 oC!
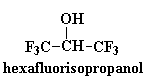
-
These polyketones have another interesting property. They won't dissolve
in much of anything. To dissolve it you have to use something really
weird like hexafluoroisopropanol. This comes in handy should you want to
make a car part out of this stuff. A car part is no good if it dissolves
when you spill gasoline on it. But that's no problem with polyketones!
This is all very nice, but really, now, what's the big deal? There are lots of high performance plastics out there. There are poly(ether sulfones), poly(phenylene sulfide), polyimides... We've seen high performance before. What makes polyketones so special?
This is what makes polyketones so special. To make polyketones, you take ethylene gas and carbon monoxide, and react them with a palladium(II) catalyst:

Ethylene is dirt cheap; it's the monomer for polyethylene. Carbon monoxide is dirt cheap, too. You make carbon monoxide every time you burn wood, or a candle, or just about anything containing carbon. The reaction is also easy to carry out. Most high performance polymers are difficult to make, because the chemistry has to be just right for the reactions to work. Cheap monomers, and easy chemistry make polyketones rather cheap, a whole lot cheaper than other high performance plastics. In time the price may come down to less than a dollar per pound! This is why we think a lot of stuff is going to be made out of polyketones in the future.
We made the prediction above before 2010 when the company marketing this polymer discontinued production. Then in 2014, surprise! A different company bought the rights to make it. Things were looking up for a while. Well, now it's 2019 and guess what? This wonderful polymer has been discontinued again!
What's strange about this is the performance to cost ratio for this polymer. Based on the extremely good properties and much lower cost than other high performance polymers, this material should be a no-brainer. Guess it comes down to company CEO's wanting a quick return on investment, and that just doesn't happen with a new polymer. It takes years, sometimes decades, before a newly introduced polymer becomes profitable, and companies now-a-days just won't wait that long. Sad for us end-users. We're stuck paying premium prices for existing polymers even though they may not be all that profitable as it is. Such is life in commercial polymer manufacturing.
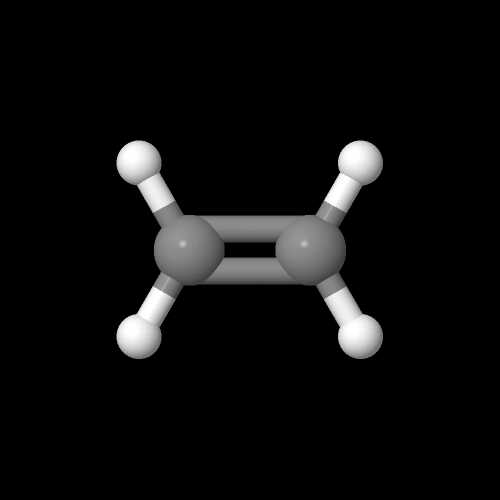
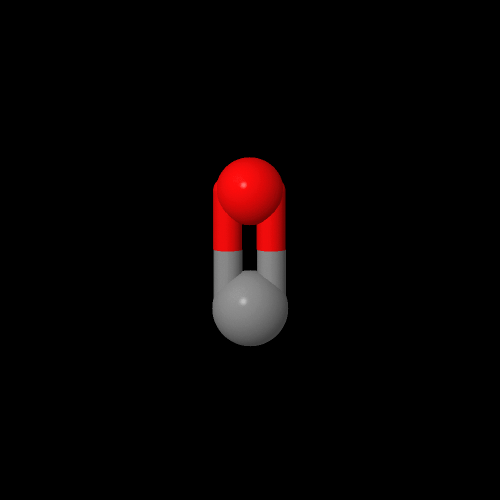
Here's some models of the monomers if you want to play with them. Ethylene is on the left and carbon monoxide is on the right. Click on the images to pop up the 3D models you can rotate or zoom in and out.
One Last Detail
There was a problem with this polyketone when Shell first made it. You see, this polymer is very highly crystalline, meaning that the polymer chains are packed together in a very orderly fashion. This makes the polyketone very strong, but the down side is that it also makes them very brittle. Chemists went to work and came up with a solution. They found that using a little bit of propylene with the ethylene and the carbon monoxide would make the polymer less crystalline.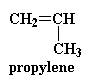
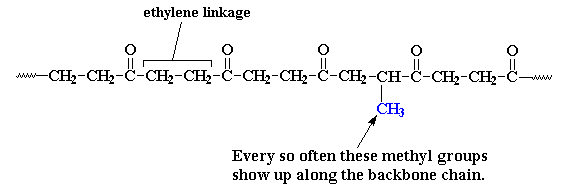
Every now and then there's an extra methyl group attached to one of the ethylene units. These methyl groups get in the way when the polymer chains try to pack into crystals. Parts of the chain can still pack, but not as well as before. The bad part is that the melting temperature drops to about 220 oC, because the crystals aren't as perfect. The good part is that the polymer is now a lot tougher, less brittle and easier to process. This polyketone made with ethylene, carbon monoxide, and a little bit of propylene is the Carilon that was marketed back in the day...
Keep watching this page. As soon as we find out what people are making out of these polyketones, we'll post something here about it!

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |