A little something about the element
FLUORINE
There is no "flour" in "fluorine"!
It doesn't even sound like "flower."
It sounds like "floor - een".

A little something about the element
| |||||
|
First, let's make sure we can spell it right! There is no "flour" in "fluorine"! It doesn't even sound like "flower." It sounds like "floor - een". |
F would really really like to have just one more electron. When F gets one more electron, it has a (-1) charge (and that makes it an ion - an anion, to be exact). (Learn more about ions here.) The symbol changes to F- and its name changes to fluoride. Sodium fluoride is added to toothpaste (and often drinking water) to help prevent tooth decay. | ||||
| The symbol for fluorine is F. (That's even easier to spell!) | F | ||||
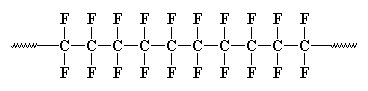
If all four H atoms in the monomer ethylene are replaced with four F atoms, the new monomer is tetrafluoroethylene. The monomer tetrafluoroethylene is:
  |
The bond between a fluorine atom and a carbon atom is extra strong - even stronger than a bond between two carbons. It's so stable that nothing will react with it, even when it gets as hot as a frying pan! | ||||
| Because PTFE is non-stick, you can fry things without grease or butter. This means less fat and cholesterol, for a healthier heart. | |||||