 |
stores energy |
| vs. | |
 |
supports & strengthens |
Did you know that the polymers starch and cellulose are both made by plants? In fact, plants make both starch and cellulose by connecting glucose molecules together. Every time they add a glucose to make the chain longer, a water molecule pops out! Add a glucose, out pops H2O! Add a glucose, out pops H2O! And so on and so on until the chains are really long. A starch chain can have 500 to 2 million glucose units. Cellulose can have 2,000 - 14,000 glucoses. That's a lot of sweetness!
A closer look at GLUCOSE
| Glucose is a funny little molecule. Glucose likes to be in a ring, but sometimes the ring opens up. (Why? Why not? You can stand up, you can sit down. So sometimes you stand up!) | When the ring closes again, the -OH can be pointed down, or it can be pointed out. Either way, it's still glucose! |
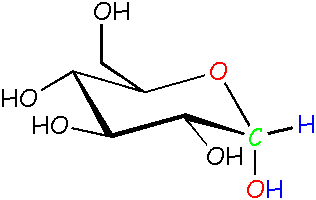 Glucose: α (alpha) form The -OH is pointed down instead of out. We didn't draw in all C and H atoms that just hang out. See this pop-up for more info about drawing structures like this.
|
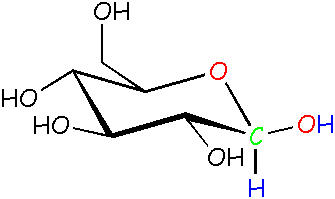 Glucose: β (beta) form See? The -OH is pointed outward instead of down. By the way, here in science land we call these molecules isomers, because they're made up of the same atoms that are put together differently. |
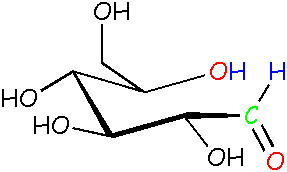 Glucose: open chain form Look at the blue H atoms. Now they're free to move around because they're not locked into the rings anymore. Compare this molecule to the ones above and the one on the right. |
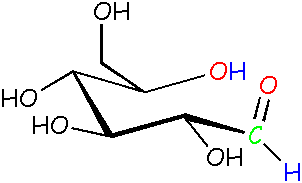 Glucose: another open chain form Compare this guy to the other open chain form on the left. It's almost the same, but one of the bonds is turned around, making the red O point up instead of down. Yep, it's now free to do that! It's like swinging your arm around. |
Now, just for fun, click your mouse over the ring-closed alpha isomer on the left above. The pop-up contains a 3D version of this isomer. Move the structure around with your mouse click on and see if you can line it up with the model that's drawn on this page. You should be able to see the OH group pointing down, no? It's a little confusing because the minimized energy for this structure takes into account intramolecular hydrogen bonding, meaning hydrogen bonding between groups within the same molecule. This makes the various OH groups on the ring point up and down rather than out from the middle. Oh, well, that's what makes chemistry so much fun!
Energy or Strength?
Starch to store energy
Plants really know how to use glucose. To make starch, they use α-glucose, with the -OH pointed down.
| That -OH is right where the next glucose will go. Since that one -OH is pointing down, it gives the chain a built-in curve. That curve is what makes starch so good for storing glucose. The starch polymer curls around and makes a nice little package. | 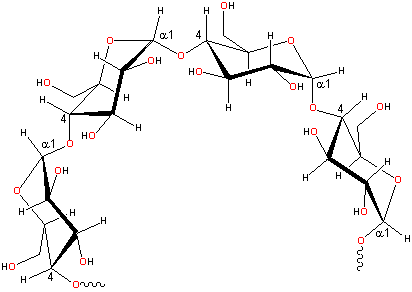 |
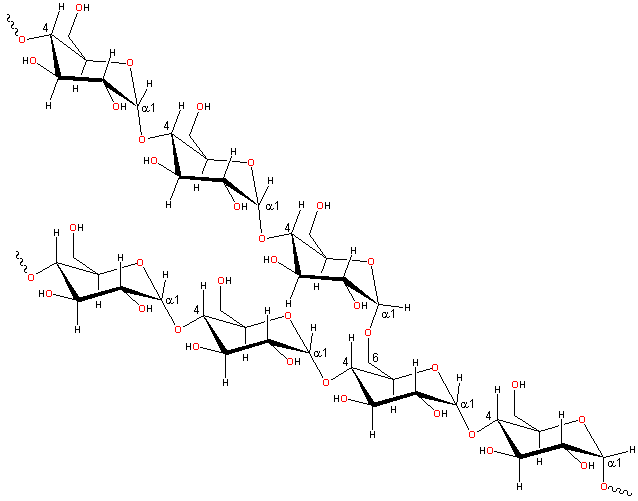 |
Many starch polymers have a lot of branches that are short chains of glucose (see picture below). When plants need energy, they grab some starch and start chomping glucoses off the ends and branches! |
Cellulose for strength
Remember that plants use cellulose to make their leaves and stems (or trunks!) strong.

To make cellulose, plants use β-glucose, with the -OH pointed out. Plants flip every other glucose over, too. I know, that's weird, but it works! It works because it helps the cellulose chains to stretch out and snuggle in next to each other. (You can read more about that on the cellulose page.) That makes cellulose fibers STRONG, strong enough to make fibers - and rope, and clothes.
Also, the chains are so close to each other, that even little molecules of water can't get in. That's good - that means that your cotton t-shirt won't wash away in the rain!
Why we eat the corn but not the cob! (But cows can eat both...)
 One more thing: You can eat starch, but you can't digest cellulose. Your body contains enzymes that will break starch down into glucose to fuel your body. (Click here for a way to taste this for yourself!) But we humans (and puppies and kitties) don't have enzymes that can break down cellulose.
One more thing: You can eat starch, but you can't digest cellulose. Your body contains enzymes that will break starch down into glucose to fuel your body. (Click here for a way to taste this for yourself!) But we humans (and puppies and kitties) don't have enzymes that can break down cellulose.
Some bacteria can digest cellulose. Animals like cows and horses and termites have these friendly little bacteria in their stomachs (or somewhere else in their digestive system). That means that when cows eat grass and termites eat wood, they can digest it with a little help from the microbes. No such luck for us, though! When we eat cellulose (and we do - all plants have some), it isn't used for energy, but it does help us. Click here to see how.
The Cellulose Page

|
Return to Kinds of Polymers |

|
Return to Main Page |