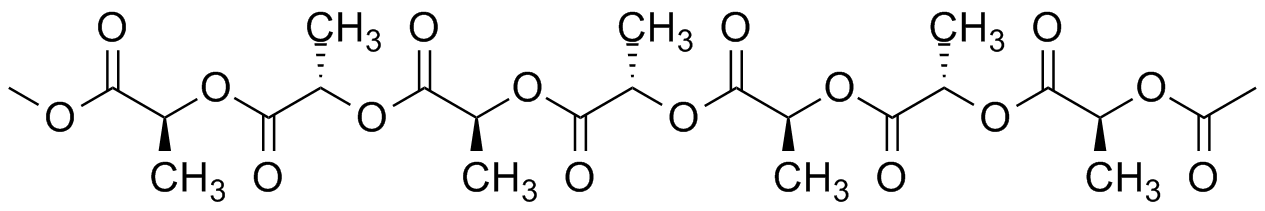
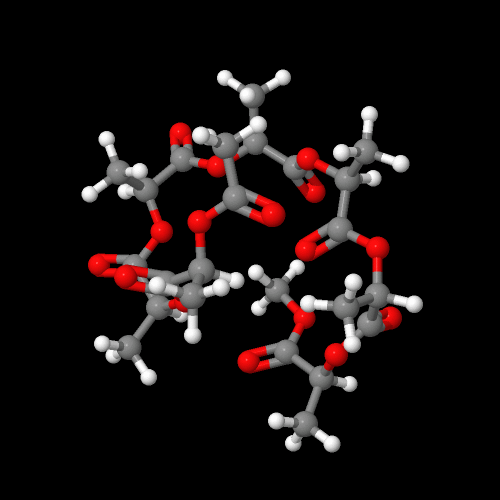
The model above is an image of the pdb model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.

Why Polyesters Anyway?
Why to make slick feeling, no-iron clothes from, right? Naw, just kidding. I actually got a polyester shirt from my mother one Christmas. Wore it exactly one time, then it ended up in the garbage. But, hey, that was an aromatic PET polyester...
Polyesters, of course, include the partially aromatic polymer, in the form of fibers, that was used back in the seventies
to make all that wonderful disco clothing, the kind you see being modeled
on the right. But since then, the nations of
the world have striven to develop more tasteful uses for polyesters, like
those nifty shatterproof plastic bottles that hold your favorite refreshing
beverages (picture below).
 One major problem with the traditional PET polyester used in so many things is
that it doesn't degrade in the ground or on the ground or in the ocean. You can
find PET articles floating around in the middle of the Pacific Ocean that have
been there for years. The follow-on problem is, animals try to eat them, by accident because "they're so shiny!" Or sometimes fish or aquataic mammals get caught in them,
and you guessed it, they meet an early demise. Not good for biodiversity.
One major problem with the traditional PET polyester used in so many things is
that it doesn't degrade in the ground or on the ground or in the ocean. You can
find PET articles floating around in the middle of the Pacific Ocean that have
been there for years. The follow-on problem is, animals try to eat them, by accident because "they're so shiny!" Or sometimes fish or aquataic mammals get caught in them,
and you guessed it, they meet an early demise. Not good for biodiversity.
A-B Aliphatic Polyesters
So inventive scientists looked around for a solution: a new polymer with properties just as good (or almost as good) but capable of biodegradation. Even better if the new polymer is directly made from all-natural materials. But hey, isn't everything in the universe already "natural?" Sure, but there's differences if you're trying to make something nature is able and willing to take back. We know that polypeptides that are major components of many plants and animals are biodegradable. Many of them have great properties we still haven't matched with our synthetic polymers. Why not make a polymer like a protein that nature already has enzymes to break down ("eat" is the operative word)? In fact, the image below is of just such a semi-natural (or is it semi-synthetic?) poly(amino acid) made from all-natural alanine. Works great and has useful properties but it's very expensive as commercial polymers go.
For various reasons, making a protein like polymer is not very easy. Nature knows how, but the best we can do makes the product polymer pretty expensive- read "nylons." Nylons cost less than poly(amino acids) but still more than most other polymers like polyethylene. Plus our synthetic versions don't degrade all that fast in natural environments, so they aren't much use for what we need here. Too bad!
But esters hydrolyse (degrade) more easily than amides! Maybe an ester analog of a protein would work great even if it doesn't have the intra- and intermolecular hydrogen bonds that give proteins (and synthetic nylons, for that matter) their great properties. Let's see where this idea gets us.
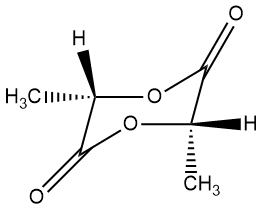
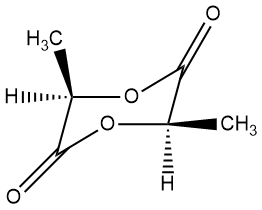
LL- or SS-lactide DD- or RR-lactide
We kind of gave the solution away at the very top. In case you don't recognize that beautiful polymer, it's called poly(L-lactic acid) or PLL for short. Not to digress too much, but you might be curious about the "L" in the name. That stands for a specific stereoisomer (or optical isomer, as it does rotate polarized light in one specific direction). The "L" isomer is the one on the left above and the cyclic dimer is called LL-lactide. The other isomer (right above, DD-lactide) is called the "D" isomer. The key difference is the tetrahedral carbon between the acid group and the alcohol group. Since that carbon has four completely different substituents attached, it's "chiral," meaning there are two stereoisomers depending on how the four groups are attached. Just so you know, the "L" isomer is also called the "S" isomer and the "D" is called the "R" version. Click on either of the images above to pop up a 3D version you can rotate and zoom in on. Anyway, we'll talk more about that in a separate page later.
So here's something neat: if you make a polyester from either pure L-isomoer or pure D-isomer, it will have exactly the same physical properties. Weird but true. The melting point and the glass transition temperature will be the same for both, exactly the same. Solubility, processing conditions, and behavior in any application that works for this polymer are all the same. The only difference is how each polymer interacts with polarized light and (here's the catch) with the other stereoisomeric polymer.
What's that you say? How it interacts with the isomer polymer? Yup, I said that, and here's why. If you mix the two polymers together at a 1:1 ratio, either in solution or in the melt, and then let the mixture solidify, the product will have completely different properties than either of the starting polymers. You might think it's because they've reacted with each other somehow, but that's not the reason. It's because the two stereoisomeric polymers form a perfect complex together, a double coil with the two different isomeric chains winding around each other in perfect harmony. (Do I feel a song coming on?).
What's Up With "Stereoisomers?"
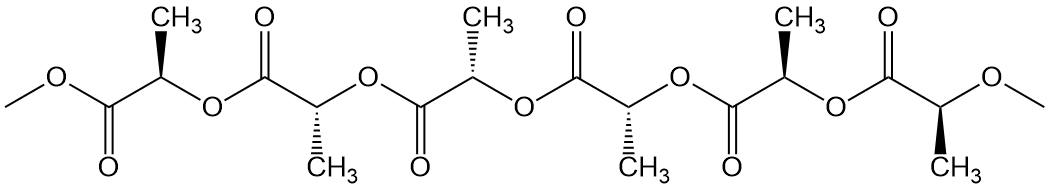
Well, then, a good question might be, are both stereoisomers of poly(lactic acid) used? And the answer is, nope. While we could make either polymer for about the same cost, remember our goal here: to make a polyester that is biodegradable. And what is involved in biodegradation? You already know: something has to digest the polymer, most probably using a natural enzyme of some kind. And guess what? Those enzymes can easily eat only the L-isomer polymer, not the other one. This digestion problem is all caused by the simple methyl group attached to the alpha-carbon (take a look again at the cyclic dimers). So what do you do with all that D-isomer you made by accident? Hope is not lost! Combine it with some of the L-version, make the D,L-lactide maybe, and polymerize that to the random copolymer shown below. Properties are a lot worse but if you need a softer, gentler polymer, that's also biodegradable, this is your baby!
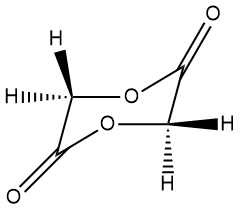
Now take a look at the different cyclic monomer below (the cyclic dimer of glycolic acid called glycolide) along with an image of its polymer. What's missing? Oh, yeah, the methyl groups! So that means that polyglycolide has some of the good properties of polylactide but differs in key ways we don't have time or space to go into right now. Let it be said, though, that it's used in specialty applications like sutures that dissolve in the body so you don't have to take them out later. Click on either of the two images to pop up a 3D version to examine in more detail.
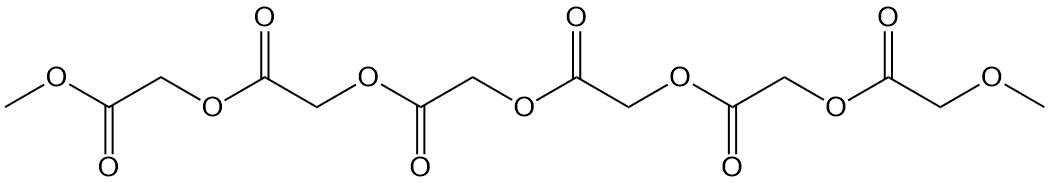
Two Other A-B Polyesters
There are two other commercial and almost-commercial A-B polyesters also made from cyclic A-B monomers. Those two are polycaprolactone and polybutyrolactone. Caprolactone is an inexpensive monomer made by the Bayer-Villager oxidation of cyclohexanone. Butyrolactone is a little more expensive but it still makes a wonderful polymer. Read on...
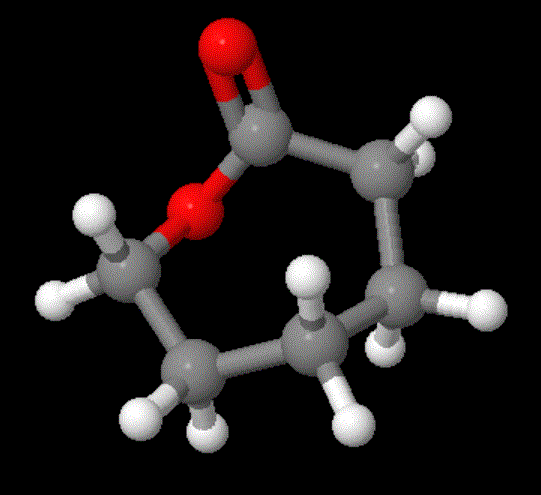
First let's talk about one of the most common and inexpensive of the A-B polyesters: polycaprolactone. No, I didn't misspell "polycaprolactam." That's a nylon or polyamide and this is a polyester.
So why is this polyester so readily available? Why is it used so much in copolymers as a flexibilizing component, especially block copolymers? Mainly because it's cheap (duh!). And now you ask, "Well, why is it cheap?" Because, of course, it's made from an inexpensive starting material (cyclohexanol which is the reduction product of phenol) using an inexpensive oxidizing agent (like peroxide or benzoylperoxide). That oxidation process results in a simple ring-expansion reaction (if you're interested, try searching for Baeyer-Villiger reaction or find an advanced organic chemistry book). Anyway, the model for the monomer, caprolactone, and polycaprolactone are right below; click on either to go to one you can play with more.
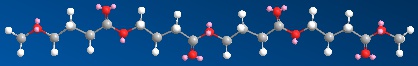
We won't spend much time on polybutyrolactone. It's useful and has its place in the pantheon of polyesters. At least we've included its 3D model that you can pull up by clicking on the image below.
Maybe we shouldn't tell you, but there (in fact) a fair number of other A-B polyesters, some simple and some more complicated. If this is an area you're interested in, let us know and we'll make a new page devoted to all these wonderful variations on this theme. Or you can try searching the internet- it's getting more and more junked up with useless ads but you might find what you're looking for. Good luck. For now let's move on to that burning question you have: see below for the answer.
You may have noticed something about all the aliphatic polyesters we've talked about so far, something they all have in common and that we've mentioned. They're all A-B polymers, meaning that each repeat unit (and monomer) have exactly one each of the two different functional groups that make up the connecting ester group. There are other commercial and almost-commercial polyesters with similar but different structures. They're called AA-BB polymers (if you hadn't already guessed).
Aliphatic Polyesters That Aren't A-B Type
Now I just bet you're all asking yourself the obvious question: If we've been talking about A-B polyesters so far, what about this other kind, whatever it is? Well, sure, but you should have already guessed that the generic representation is something like A-A/B-B or AA-BB. This implies that the two functional groups needed to make the ester units are on two different monomers with two each alcohols on one and two carboxylic acids on the other. If you guessed this, you'd be 100% right.
Let's get down to the nitty-gritty of such materials. Well, not so much the deep-down-details but more like a surface glance. For now we'll just list some of the names along with monomers, show you a few structures and state what's most important about these polymers. And that most important thing is that most of them are biodegradable and many are made from renewable raw materials.
So much for the good news. The bad news is simply that they cost more than the A-B polymers above, and lots more than good old PET. Add to that the fact that their properties are a lot lower (meaning worse), and most of these polymers don't have much of a market share. Oh, well, things change, and maybe the desire to be more eco-friendly will grow in us all as we continue to use up the non-renewable resources of planet earth (you know, I hope, we only get one of those?). Anyway, on with the show!
AA-BB Polyesters and Copolyesters

Other polymers used as plastics:| |
Other polymers used as fibers: | |
| Polypropylene | Polypropylene | |
| Polyethylene | Polyethylene | |
| Polystyrene | Nylon | |
| Polycarbonate | Kevlar and Nomex | |
| PVC | Polyacrylonitrile | |
| Nylon | Cellulose | |
| Poly(methyl methacrylate) | Polyurethanes |

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
