


Proteins are one of many types of natural polymers, and they are one of the most versatile by far. You name it, proteins do it. Proteins called enzymes make certain chemical reactions in your body take place up to a million times faster than they would without them. A protein called hemoglobin is found in blood and carries oxygen from your lungs to your cells. Another protein called collagen is a strong and tough material that makes up your skin and fingernails, and even helps to hold your internal organs in place.
Funny you should ask, because we can. Thinking about proteins (also called "polypeptides") makes you realize that the most important part is the amide group in the backbone chain. In fact, that makes these polymers very similar to another family of synthetic polymers, the nylons. Want to see an amide group so you know just what it is I'm rambling about? Here's an amide group, and below it is a polyamide:
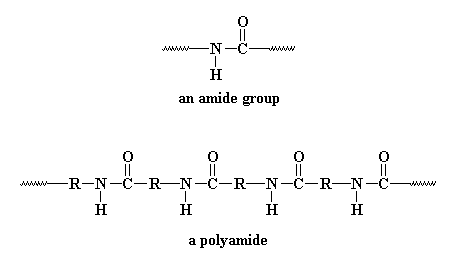
That R you see in betwixt the amide groups can be lots of things but usually is an aromatic ring (in the aramids) or a polymethylene chain. In proteins, the R is a single carbon atom, with two pendant groups attached. One pendant is a hydrogen atom, always, and the other is a wild card. It can be a lot of different things. In this picture we just represent it as R'.
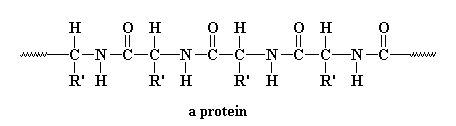
In a living creature, enzymes (made of amino acids in a protein) link up the amine and carboxylic acid groups to form the amide links:
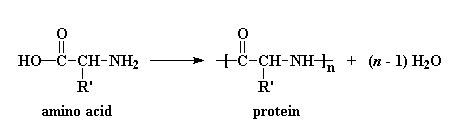
In the lab we use a special type of monomer actually made from one of the amino acids. That monomer is called an N-carboxyanhydride or NCA for short.
Here is the image of the alanine NCA:
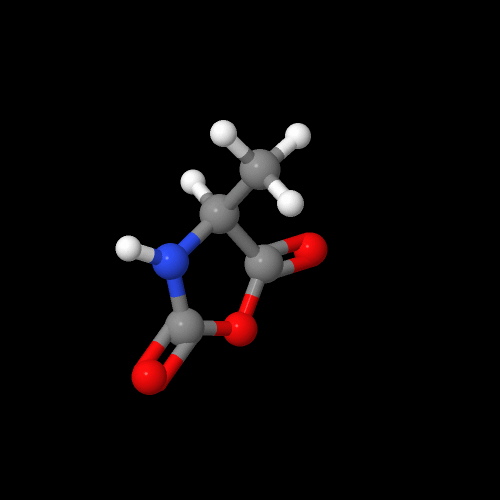
The model above is an image of the pdb model you can view by clicking here
or you can just click on the image itself.
Be sure to close the new window when you are ready to come back here.
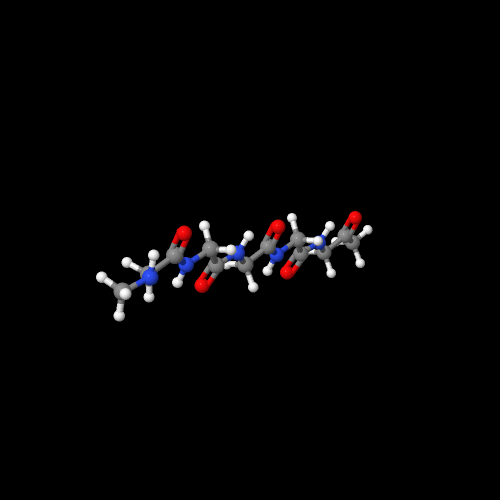
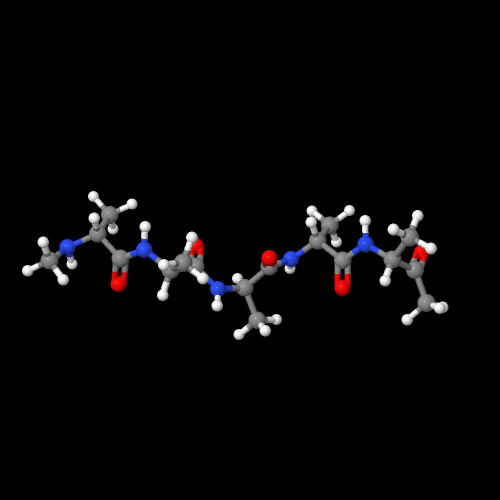
The model on the left above of polyglycine shows that it really is a nylon like nylon 6.
This type looks like it's made from a monomer with an amine on one end, a carboxylic acid
on the other end and several methylenes in between. Polyglycine has only one methylene and is
therefore the simplest A-B nylon that you can make.
The two 3D models show an important characteristic of polypeptides and of natural proteins as well. That is, there are two common types of 3D structures that enormously impact the physical properties. Polyglycine has an almost perfect zig-zag molecular structure that allows the chains to pack in what's called a "beta-sheet" form. Compare that to the 3D structure of polyalanine. It forms a helical structure (play around with the actual model to see this better). That structure is called an "alpha-helix" and it's very important in controlling how functional groups in a protein can interact. In enzymes, that means it can do their job by working together, but in silk (lots of beta-sheets) we get a silky feel (sorry) that is hard to imitate with man-made polymers.
Want to know something else? Proteins also make up silk. Silk is such nifty stuff that scientists tried to make synthetic silk. They tried to make synthetic polyamides, and what do you know, they did it! The artificial polyamides are called nylons.
You want more natural polymers? Then check out these:

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
