

Keywords
electrolyte,
ion,
random coil.
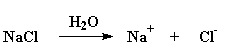
Polyelectrolytes are polymers that do the same thing. They fall apart in water. Want to see one? How about polyacrylic acid? Put this stuff in water and the acid hydrogens split off with the water molecules and form H3O+ ions, like this:
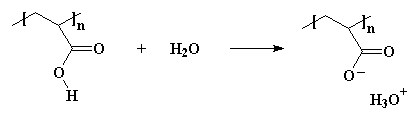
But when the polymer chain is covered with negative charges (which repel each other), the polymer can't be bunched in on itself. So the chain stretches out, like this:
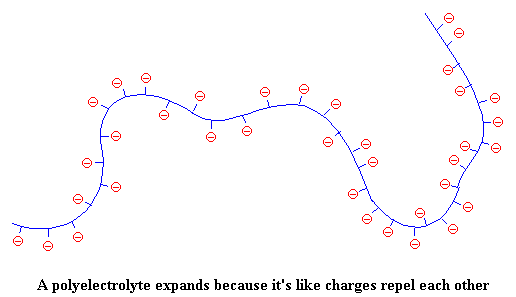
This makes the solution (remember we're talking about polyelectrolytes in solution) more viscous. Think about it. When the polyelectrolyte chain stretches out it takes up more space, and is more effective at resisting the flow of the solvent molecules around it. So the solution becomes thick and syrupy.
But there's a way you can stop this from happening. If one takes a solution of a polyelectrolyte in water, and throws in a lot of salt, something fun will happen. The NaCl will separate into Na+ and Cl- ions. In the case of a negatively charged polyelectrolyte like poly(acrylic acid), the positively charged Na+ ions will get in-between the negative charges on the polymer, and cancel them out in effect. When this happens, the polymer chain collapses back into random coil again.
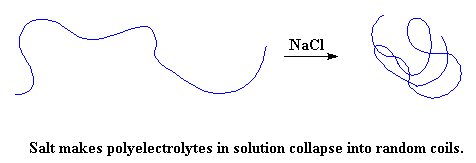
What? You don't believe me? Remember I said this was something you could do. And now I'm going to tell you how. Take some hair gel and put a big glob of it in a bowl. Now take a salt shaker, and pour on the salt like you were trying send your grandmother's blood pressure through the roof. When you do this, the gel will collapse into a pretty boring ordinary liquid. This is how dramatic the change in viscosity is when the polymer chains collapse!

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |
