

Structurally, it's a vinyl polymer, and is similar to polyethylene, only that on every other carbon atom in the backbone chain has a methyl group attached to it. Polypropylene can be made from the monomer propylene by Ziegler-Natta polymerization and by metallocene catalysis polymerization.
This is what the monomer propylene really looks like:
Research is being conducted on using metallocene catalysis polymerization to synthesize polypropylene. Metallocene catalysis polymerization can do some pretty amazing things for polypropylene. Polypropylene can be made with different tacticities. Most polypropylene we use is isotactic. This means that all the methyl groups are on the same side of the chain, like this:
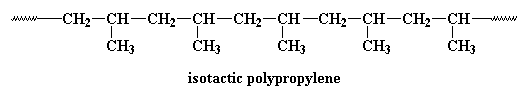
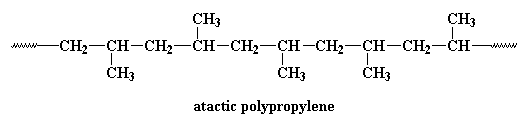
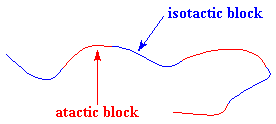
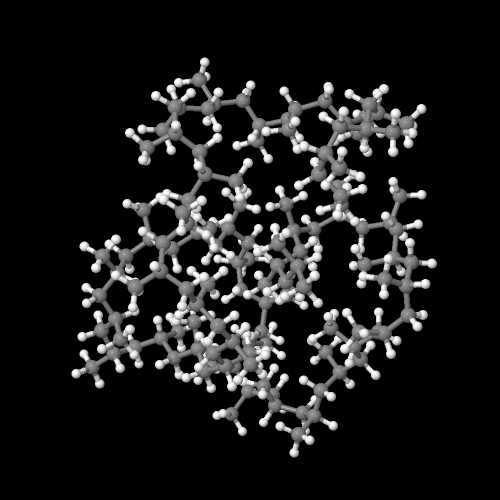
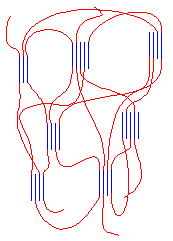 This polymer is rubbery, and makes a good elastomer. This is because the isotactic blocks will form crystals by themselves. But because the isotactic blocks are joined to the atactic blocks, the little hard clumps
of crystalline isotactic polypropylene are tied together by soft rubbery tethers of atactic polypropylene, as you can see in the picture on the right.
This polymer is rubbery, and makes a good elastomer. This is because the isotactic blocks will form crystals by themselves. But because the isotactic blocks are joined to the atactic blocks, the little hard clumps
of crystalline isotactic polypropylene are tied together by soft rubbery tethers of atactic polypropylene, as you can see in the picture on the right.
To be honest, atactic polypropylene would be rubbery without help from the isotactic blocks, but it wouldn't be very strong. The hard isotactic blocks hold the rubbery isotactic material together, to give the material more strength. Most kinds of rubber have to be crosslinked to give them strength, but not polypropylene elastomers.
Elastomeric polypropylene, as this copolymer is called, is a kind of thermoplastic elastomer. However, until the research is completed, this type of polypropylene will not be commercially available.
The polypropylene that you can buy off the shelf at the store today has about 50 - 60% crystallinity, but this is too much for it to behave as an elastomer.
| Other polymers used as plastics include: | Other polymers used as fibers include: |
| Polyethylene | Polyethylene |
| Polyesters | Polyesters |
| Polystyrene | Nylon |
| Polycarbonate | Kevlar and Nomex |
| PVC | Polyacrylonitrile |
| Nylon | Cellulose |
| Poly(methyl methacrylate) | Polyurethanes |

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
