

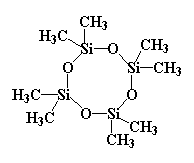

You can see what it looks like in 3-D by clicking here or on the image above.
Be sure to close the new window to get back here.
This molecule does something fun with bases like NaOH. A hydroxide group will donate a pair of electrons to one of the silicon atoms in the ring, which is all too happy to accept. The only problem with this is that the silicon already has its fair share of electrons, that is, eight. It can't have ten now. So it has to get rid of a pair. The pair that gets ditched is the pair that makes up the silicon-oxygen bond. So the pair is shifted entirely to the oxygen.
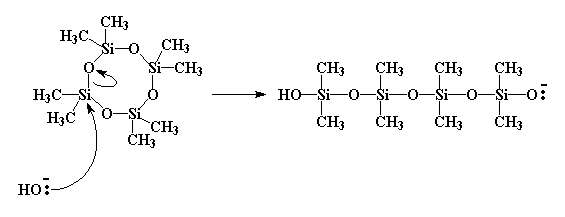
Click here to see a movie of this reaction.
This breaks the bond between the oxygen and the silicon. Yes, I know this bit of information comes straight out of the latest issue of the Journal of the Blatantly Obvious, but I bring it up for a reason. When this bond is severed, the ring is no longer a ring. It has been opened.
And furthermore, the oxygen that gained the pair of electrons now has a negative charge. It can attack a second monomer molecule, exactly like the hydroxide attacked the first.
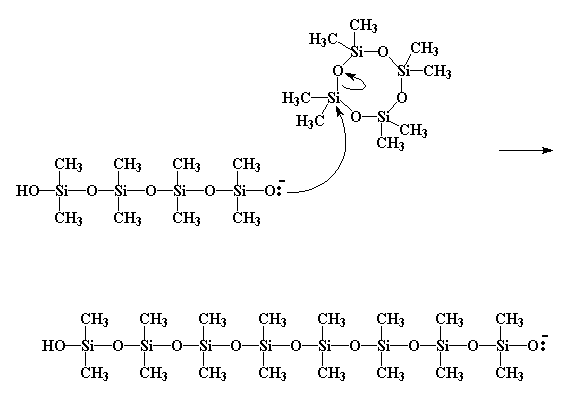
I think we can all see where this is heading. More monomer adds, and eventually, we get a brand new polysiloxane chain.
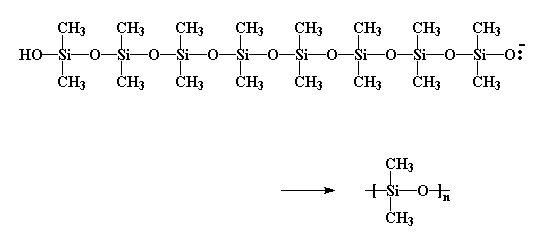
Because we open monomer rings to make the polymer, this is, of course, a ring-opening polymerization.

|
Return to the Silicone Page |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
