Poly(adamantyl methacrylate)
One example of the "adamantyl effect"
Use your mouse on the model to rotate and zoom.
Click items below for other modifications.
spin on spin off
spacefill wire thick wire ball&stick
dots: Vanderwaals dots off
Poly(adamantyl methacrylate) is just one polymer with an adamantane group attached. (For more information about adamantane, click here). The monomer for this polymer is easily made from adamantanol and a reactive methacrylate reagent, similar to the way the acrylate monomer is made below, with X being chloride or the anhydride of the acrylate. And just to ease your mind, adamantane can be made easily from dicyclopentadiene, which is itself pretty inexpensive, and then readily converted to the alcohol.
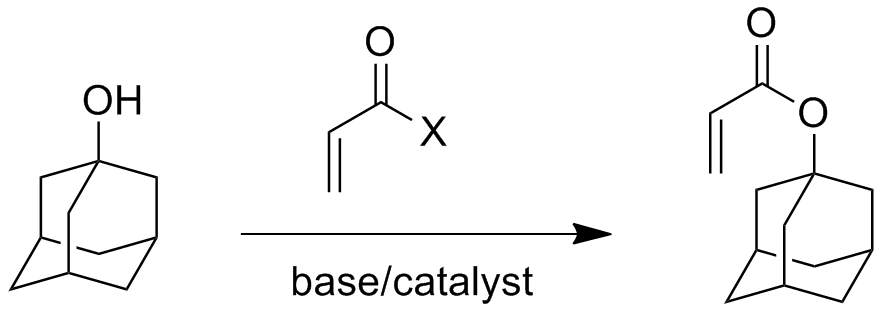
Both polymers are very unusual for acrylate and methacrylate ester polymers. The acrylate polymer has a glass transition temperature of over 150 oC, well below it's decomposition temperature but one of the highest Tg values of any acrylate polymer I know about. The methacrylate polymer, on the other hand, has a glass transition temperature that is actually higher than its thermal decomposition temperature (which is around 254 oC). This means it's almost impossible to melt process this polymer, since it never softens enough before it falls apart. Bummer! Also, the polymer made by radical polymerization (how you'd normally do it for a commercial material) has no crystalinity, which means that it has no melting point. Good thing, actually, since Tm is always higher than Tg, and Tg for this polymer is higher than Tdec. Logic tells us that Tm would be much, much higher than Tg, but then, who cares? It's not crystalline anyway, which does lead to other interesting properties, like better solubility.
Let's think a little about why not being crystalline matters here. Lots of polymers like nylons, PET polyester, polypropylene and good old polyethylene get their good thermal properties from crystalline domains. Those domains act like physical crosslinks, keeping the rest of the polymer, the amorphous part, from moving around, soft and gooey like (see figure below). That amorphous, non-crystalline part is usually more than half to two-thirds of the total polymer so how it behaves is really important. It's even more important for polymers that don't have those crystalline crosslinks to hold everything in place.
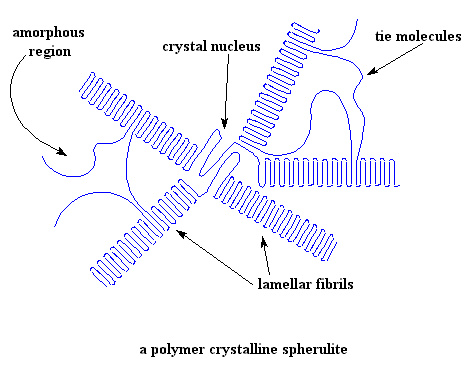
And just to remind you, while the melting transition involves long polymer chain segments freeing themselves from the crystalline lattice (at around the crystalline melting point, Tm), the amorphous phase undergoes a different type of thermal transition. It's called the glass transition, and it's always much lower then Tm. The molecules go from a glassy state (hence the name) to a rubbery or liquid-like state as it passes through Tg. The molecular motion associated is very different than for Tm, consisting of only short-range reorientations that involve just a few repeat units. Not only do those short-range motions make the polymer flexible, it usually makes it permeable to gases, maybe even to liquids to some degree.
Raising the Tg of the polymer can have positive impact on properties. For example, if Tg is way above the use temperature, whether that's room temperature or the high temperatures under the hood of a car, the polymeric material won't soften and distort. Thermally molded polymers (most of those used commercially) are cooled down after molding. This locks in whatever residual strain might be present from doing the molding and cooling as fast as possible. Remember, time is money, so the faster you make your part and get it off the molding machine, the more money, right? Problem is, if you warm the sample back up to near the Tg, the locked-in strain will cause it to distort, maybe shrink or even soften to the point it can't hold its shape at all. Bummer! You just lost a good customer.
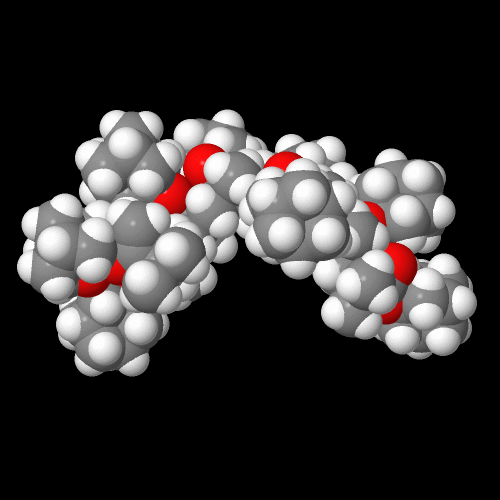
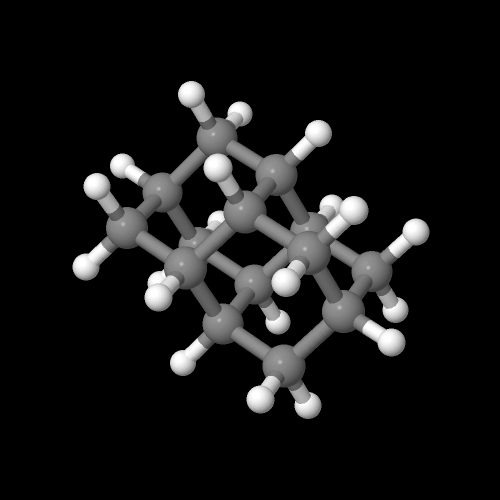
Take a look at the 3D model of this polymer at the top of the page. Play around with it, rotate it, maybe zoom in or out so that you can see how parts of the chain are arranged. This is an atactic version normally obtained with free radical polymerization. The lack of regular arrangement of the pendent groups down the polymer backbone is the main reason this polymer is non-crystalline. You can do the same molecular visualization for adamantane itself by clicking on the image below of this highly symmetrical C10 saturated hydrocarbon.
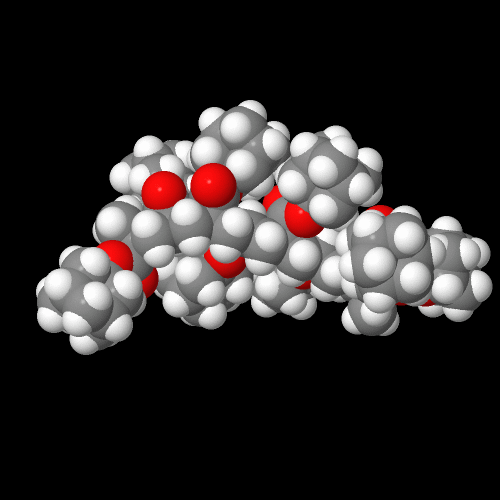
Below are the space-filling models for poly(adamantyl acrylate) on the left and poly(adamantyl methacrylate) on the right. I hope you're as impressed as I am by how humongous the pendent adamantyl groups are. In fact, they overshadow the size of the backbone atoms. No wonder the effect of adamantane on Tg is called the "boat anchor effect." On the molecular scale, it's a pretty big boat anchor!
The researchers who evaluated kinetics of radical polymerization for monomers like the adamantyl acrylate and methacrylate found something totally surprising. You might think that with the "boat anchor" attached to the monomer, the rate of polymerization would slow down. After all, that anchored monomer has to wend it's way to the growing chain end so it can react. You'd be wrong in thinking this, however, because of the "rest of the story." Turns out, you also have to consider radical termination, which requires two radical chain ends coming close enough to react with each other. Now the boat anchor really plays a role as it sterically blocks that from happening. So what? you ask. Well, here's what: if termination rate goes down, radical concentration goes up. Now since the rate of propagation depends on both the rate of addition of monomer to the radical chain end and the radical concentration, the overall rate actually increases compared to simpler, less hindered monomers. Who'd of expected that!
Another interesting fact about these polymers is their solubility. Sure, they're completely amorphous, which helps, but even more helpful are those big (shall we say giant?) hydrocarbon groups hanging off the chains. Lots of solvents like to interact with them, especially common ones like chloroform and toluene. So even if you can't thermally process these babies easily (well, maybe the acrylate, but I haven't seen that done yet) you can certainly spin fibers and cast films from solutions of the polymers. That would certainly be interesting although it hasn't been done yet to my knowledge. And to generalize, this greatly increased solubility of polymers with pendent adamantanes makes most or all of them dissolve readily in simple organic solvents. High Tg's and great solubility buys you a lot.
Well, you might think, if one adamantane is good, two or more must be even better, right? Yes and no, as it turns out. One example, the diadamantyl methacrylate, has been made (see the Sinkel, et.al article). It has even higher Tg than the one we've been talking about, with no observable transition up to the decomposition temperature. But what actually does that buy you? We're already above the decomposition temperature with just one adamantyl group, so going to even higher Tg doesn't change anything, except... and there's always a "but" of some kind, isn't there? (Click on the image if you want to play around with the structure of diadamantane).One thing the larger diadamantyl gives you is somewhat faster polymerization. Based on the discussion above, this makes sense. A second thing it buys you is greater reactivity with methyl methacrylate (MMA) in copolymerization. For that, I have no good explanation. And last, you don't need the homopolymer of the diadamantyl monomer when you can use a little bit in a copolymer. Even at 14% by weight with MMA, Tg jumps 20 oC or so, meaning that even a little of this "pixie dust" buys you a lot.
Turns out that Tg also depends on the total molecular weight of the polymer to some extent, but only at the low MW end. For the adamantyl methacrylate polymer at just a few thousand daltons, the Tg is already around 200 oC. It increases rapidly with MW until at 20,000 or so it's approaching the decomposition temperature (see the Fuchise, et.al article below). Of course, this is for polymer made with living radical polymerization which gives a much narrower polydispersity than obtained with normal radical polymerization. Not sure how that effects Tg but I'm sure it does somehow. My guess is that a broader polydispersity would also broaden, perhaps even lower, the measured Tg.
Now the adamantyl effect is pretty general. Put one somewhere on just about any polymer backbone, and all the things we talked about above happen: Tg and solubility go up dramatically and other properties are effected such as transparency, susceptibility to photochemical and acid-catalyzed reaction, and other things we'll not discuss now. A recent literature search (in scholar.google.com) turned up dozens and dozens of polymers that were made with pendent adamantane groups. These ranged from polymers with simple backbones like vinyl and oxyethylene to nylons, aramids, PEEK and polyimides, to name a few. All of them showed significant increases in Tg, usually around 100 oC or so compared to the unsubstituted versions. Most showed improved solubility as well. For specific applications such as in gas separation membranes, pendent adamantanes often increase separation efficiencies also.
So there you have a novel and effective property modification that can be used on virtually any polymer you might be interested in. Why not
try the adamantyl effect on some polymer you know and love but want to be just a little bit better (or a lot even)?
References, to name just a few:
Matsumoto, A., Tanaka, S. & Otsu, T. Synthesis and characterization of poly(1-adamantyl methacrylate): effects of the adamantyl group on radical polymerization kinetics and thermal properties of the polymer. Macromolecules 24, 4017-4024 (1991).
Otsu, T., Matsumoto, A., Horie, A. & Tanaka, S. Synthesis of thermally stable vinyl polymers from adamantyl-containing acrylic derivatives. Chem. Lett. 1145-1148 (1991)
Acar, H. Y., Jensen, J. J., Thigpen, K., McGowen, J. A. & Mathias, L. J. Evaluation of the spacer effect on adamantane-containing vinyl polymer Tg’s. Macromolecules 33, 3855-3859 (2000)
Fuchise, K., Sone, M., Miura, Y., Sakai, R., Narumi, A., Sato, S., Satoh, T., Kakuchi, T. Precise synthesis of poly(1-adamantyl methacrylate) by atom transfer radical polymerization. Polym. J. 42, 626-631 (2010)
Sinkel, C., Agarwal, S., Fokina, N.A., Schreiner, P.R. Synthesis, Characterization, and Property Evaluations of Copolymers of Diamantyl Methacrylate with Methyl Methacrylate. J. Appld. Polym. Sci., 114, 2109-2115 (2009)