Polyoxymethylene POM
aka Polyformaldehyde or Polyacetal
or even Polytrioxane
Use your mouse on the model to rotate and zoom.
Click items below for other modifications.
spin on spin off
spacefill wire thick wire ball&stick
dots: Vanderwaals dots off
Look closely at the model above. Click on it, move it around some, then zoom in so it's really big. Rotate this enlarged view around and you should begin to see the helical shape of the interior part of the model shown. This structure, a 2:1 helix it's called, has enormous importance in understanding the solid state properties of this wonder material. "Wonder material?" you ask? Sure, but right now it's the "look at it and wonder what kind..." Let's talk more.
So What's the Big Deal About POM?
You've heard of formaldehyde, I'm sure- the stuff used to preserve dead creatures for biology lab. It was also used to preserve deceased humans at one time, a different kind of dead creature, I guess. This noxious smelling liquid (actually a gas dissolved in water) is not what you would pick to make a polymer from.
But hold your horses (or cows or whatever living creatures you might possess)! It actually is used as a monomer, at least for some of the POM made today (and POM, of course, is polyoxymethylene).
But wait again, POM is unique not just in what it's made from, or for its unusual properties (we'll get to those). It's most unusual for having the simplest repeat unit chemical formula of any polymer made: one carbon, one oxygen and two hydrogens. That's two less hydrogen atoms than ethylene, the repeat unit monomer for the hydrocarbon polyethylene, the most widely made and used polymer of any kind. So POM is unique for two reasons, making it another double first place winner (something to be proud of in the world of polymer science).
One synthetic method for POM actually does use formaldehyde but it's more complicated than that because that polymer isn't stable! So read on to hear the "rest of the story" and to find out all about the unusual syntheses and properties of this POTM.
"Make it as simple as possible, but no simpler-" Albert Einstein
First of all, you should notice that there's not a lot to the repeat units of this polymer. In fact, other than polyethylene (with only two carbons and four hydrogens per repeat), it's the simplest heteroatom polymer- only one oxygen, one carbon and two hydrogens. And even with such a simple composition, this polymer has wonderful properties we'll get to in a second. Just to whet your whistle (as it were), here's a novel fact: this polymer melts around 178 oC which is higher even than Spectra polyethylene, Tm of only 150 oC. Well, why not? It's got a more polar group in the backbone at every other position- and polarity often increases intermolecular interactions, those forces that effect crystalline melting.
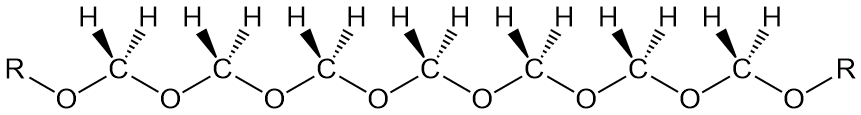
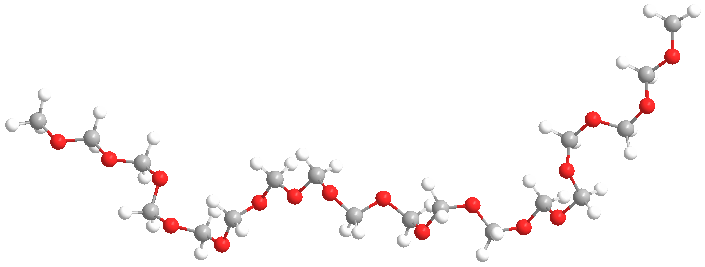
What's in a Name??
The second thing to note about this polymer is it's name, or rather names. One I gave above is "polyoxymethylene." This name reflects an IUPAC-type approach in which the name explicitly indicates what the chemical composition and linkages are. Pretty simple name for this polymer, but the IUPAC approach just doesn't work for most polymers. Take PEEK, or as it's known in the industry, "poly(ether ether ketone)." Dumb name. You can't have an ether or ketone group all by itself, meaning each of the three must be linked to something else, but what? Not to belabor the point, but the IUPAC name would be something like "poly(oxy-1-4-phenylene-oxy-1,4-phenylen-carboxy-1,4-phenylene)." Quite a mouthful, and as you might guess, no one really calls it that. "PEEK" is so much less cumbersome and everyone knows what it stands for anyway (well, everyone who happens to be a polymer scientist).
So while we could call POM "polyoxymethylene" without too much tongue-twisting, we don't. As any English major will tell you, language follows usage. It's not what the rules dictate, but what people actually say. And in polymers, the convention has grown to be accepted now of just using the monomer in the name (although that doesn't work for PEEK, does it?).
For POM, then, one name would be "polyformaldehyde," indicating that the polymer is made from formaldehyde (duh!). But here's a weird thing: while one company actually DOES make the polymer from highly purified gaseous formaldehyde, they don't call it that. Instead, for reasons that aren't clear at all, they call it "polyacetal." Well, sure, the C-O-C-O segements are technically acetals (or more correctly, formals, pronounced "for-mal" and not "formal"). But most organic chemists might think it's an "acetal" linkage which would have a pendent methyl group on every carbon. THAT polymer is much more difficult to make and not very useful anyway. Ok, then, "polyformaldehyde" is used along with "polyacetal."
Ok, ready for another weird name for this wonder polymer? How about "poly(methylene oxide)"? This name was actually used in a couple of published articles about properties of the polymer, so apparently the referees and editors of those journals thought it appropriate. Problem is, it leads to confusion on just what the monomer is. Poly(ethylene oxide) (aka polyoxyethylene) is probably the second most-simple heteroatom polymer. It's usually made from ethylene oxide, which is a three-membered ring epoxide. Ok, then, the "oxide" is consistent with an epoxide monomer, but not a formaldehyde in which there's a carbon-oxygen double bond. Oh, well, once you choose the path of "I'll name it whatever the heck I please," then all bets are off.
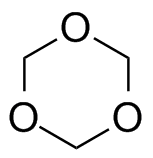
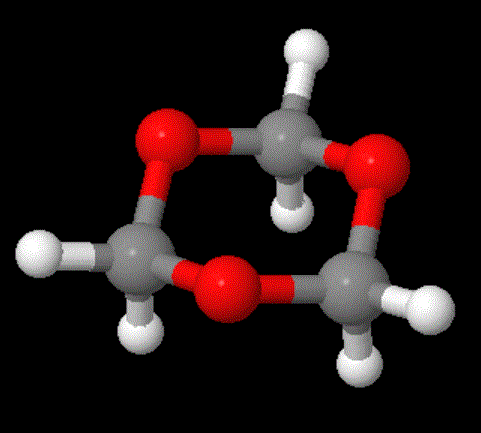
Now for the really interesting name: "polytrioxane." Whoa!" you might exclaim, "That indicates the monomer is a cyclic six-membered ring with three oxygens evenly spaced between three carbons." Right you are! So take a look at the two images above; click on the right one to pop up a 3D version you can examine more.
Turns out, the stable pure form of formaldehyde is not the CH2=O species. It's just not a happy camper all by itself: it really, really wants to bond with others (like some social clingers we all know). Well, in fact, the gas form does just that and becomes the cyclic trimer shown above. Bingo! This name (polytrioxane) works great because it accurately reflects how the polymer is made from trioxane using a different process than the one using formaldehyde.
Your head spinning yet? And over the simplest heteroatom polymer known? Ready for more spin that will make your eyes twitch and your hands move in strange magical ways? Here it is: this polymer, by whatever name you want to call it, is totally NOT stable!
Stability and Termination
"What's that you say?" you ask in total perplexity. "I thought you said that this was a commercial polymer made by two different methods. If it's commercial, how can it not be stable?"
Yeah, well, stability is a relative thing, isn't it? Put a book on the table and it's stable, but not really. It really wants to fall to the floor. Slide it to the edge of the table, and "Whap!" down it goes. Some polymers are like that. Stable under some conditions, not so much under others. We make a big deal out of this in medicine where we use polymers that work great for a while in the body, and then they decompose or hydrolyze and disappear. Dissolving sutures are like that (polylactide or polyglycolide). But here, well, with this polymer, we want to use it for pretty demanding applications. So it not being stable is a big deal.
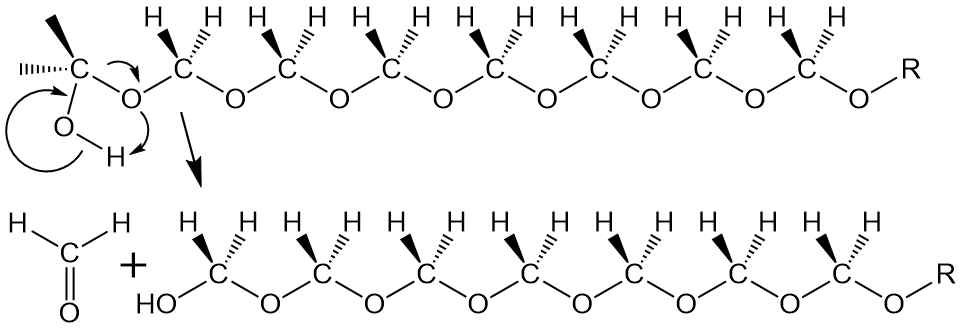
Not to make too much of this, but it so happens that pure homopolymer, with only formaldehyde repeat units, has a terminal OH group. All by itself, this group back-bites the polymer and either falls off as formaldehyde gas (above) or, if it bites back far enough (see below), it forms trioxane that we talked about earlier. What to do, what to do!
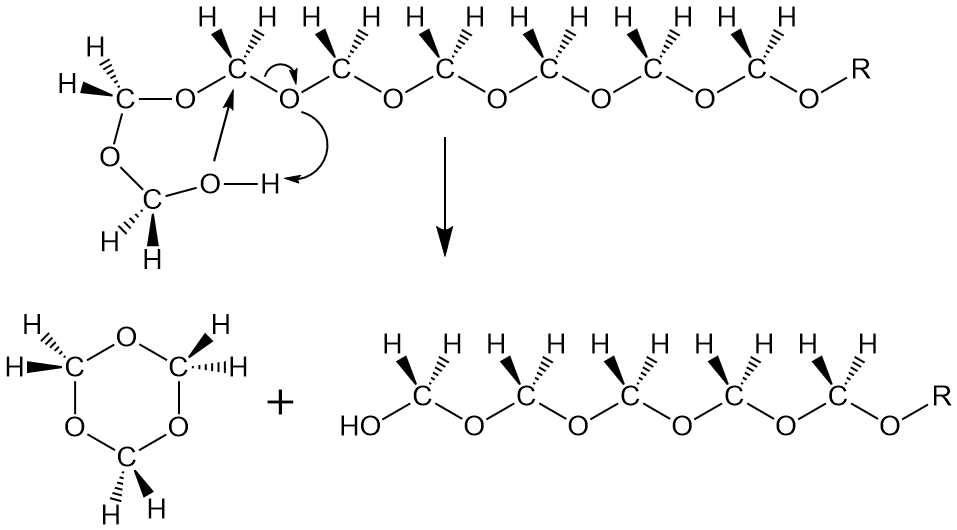
Simple: block that terminal OH group. Two different commercial processes do this in two different ways. One uses acetic anhydride to react with that OH group after the polymer is made. And as you know (or should know), that converts it into an acetate, which is a lot more stable than the OH group it came from. So this termination reaction makes the POM stable enough to process and use by making an acetate group at both ends of the polymer (top figure below). This process is apparently used by DuPont to make their POM called Delrin.
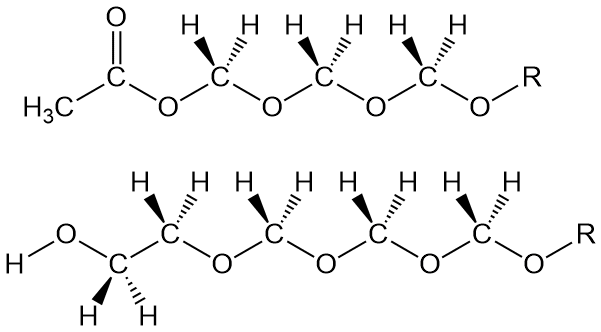
"OK, then, what about the other way? You mentioned two." Sure, it's kind of the same thing only different (isn't that just like life in general?). In this approach, the chain blocking group is incorporated into the polymer backbone DURING polymerization. The comonomer is ethylene oxide at low amounts. That incorporates oxyethylene groups into the polymer. Now when decomposition occurs to release some formaldehyde (which it does), the depolymerization stops when it hits an oxyethylene segment. That -CH2-CH2-OH group at the polymer chain end (see above drawing) is stable enough to allow the copolymer (mostly POM segments) to be processed and used. Of course, there are some additional oxyethylene segments throughout the chain structure, and this does decrease properties like Tm somewhat. Even so, it still has great properties.
So, two solutions to the same problem using two different chemistries to achieve the same result- stable POM. Oh, and this version of POM was first introduced by Celanese in the early 1960's and is called Celcon. DuPont's version came on the scene in 1960 and is called Delrin. Seems there are several other suppliers of POM and they each have their special trade name for the polymer. Forgot to mention the fact that trade names tell you exactly nothing about what the polymer being sold is actually made of. So much for names and what they mean.
And Now (drum roll, please) What's It Used For??
 Ah, the nitty gritty (whatever that is)! Turns out, POM is one of those "invisible" polymers that doesn't get much publicity despite it's important applications. It has the interesting property of having low surface friction. Combine that with thermoplastic molding, and you have a material used in all kinds of metal-on-plastic contact areas, like bearings in a washing machine. Think about all the small, repetitive motions in which some kind of metal rod rubs against a plastic support. Take copy machines: lots of POM bearings used in those. They last long enough to keep the printer cycling through all your copying needs but without the expense of traditional metal bearings like those used in bicycle wheels and on car axles.
Ah, the nitty gritty (whatever that is)! Turns out, POM is one of those "invisible" polymers that doesn't get much publicity despite it's important applications. It has the interesting property of having low surface friction. Combine that with thermoplastic molding, and you have a material used in all kinds of metal-on-plastic contact areas, like bearings in a washing machine. Think about all the small, repetitive motions in which some kind of metal rod rubs against a plastic support. Take copy machines: lots of POM bearings used in those. They last long enough to keep the printer cycling through all your copying needs but without the expense of traditional metal bearings like those used in bicycle wheels and on car axles.
 Remember Einstein's quote up at the top of this page? Another version might be, "Make it as cheap as possible but no cheaper." Meaning, of course, that price is not the only driver- the part has to perform adequately as well. Problem is, in modern manufacturing, too many think that price and cost are the ONLY important variables. Making something so cheap that it doesn't actually work, or doesn't work for as long as it should, lies on the path to self-destruction: consumers stop buying your products. Here's a mantra we should all remember: "Value equals performance divided by cost." Think about that in all the stuff you buy, especially the stuff you throw away too soon.
Remember Einstein's quote up at the top of this page? Another version might be, "Make it as cheap as possible but no cheaper." Meaning, of course, that price is not the only driver- the part has to perform adequately as well. Problem is, in modern manufacturing, too many think that price and cost are the ONLY important variables. Making something so cheap that it doesn't actually work, or doesn't work for as long as it should, lies on the path to self-destruction: consumers stop buying your products. Here's a mantra we should all remember: "Value equals performance divided by cost." Think about that in all the stuff you buy, especially the stuff you throw away too soon.
So this month's "POTM" is unique for three reasons, making it a triple first place winner (whatever that means in real life). First, it's the simplest heteroatom containing polymer. Second, it has at least three, maybe four or even five, names, depending on how you count. And third, the polymer is inherently unstable and has to be "tricked" into being stable enough to use. Neat, huh?
References, to name just a few:
DuPont product brochure for Delrin POM; found on 1-24-2019 at http://www.complast.com/delrin/DelrinData.pdf
Celcon POM information found on 1-24-2019k at http://www.craftechind.com/plastic-materials-close-polyoxymethylene-pom-acetal-delrin-celcon/
Delrin molding guide and product information found on 1-24-2019 at: http://www.dupont.com/content/dam/dupont/products-and-services/plastics-polymers-and-resins/thermoplastics/documents/Delrin/Delrin%20Molding%20Guide.pdf