monomer
This is the page devoted to a little introduction of how polymers are made. A chemical reaction that makes polymers is called a polymerization. There are many of these reactions, and they come in all kinds. But all polymerizations have one thing in common: They all start with small molecules, and join them into big giant molecules. We call the original small molecules monomers. But they can join in many different ways. People like to categorize things, so Immanuel Kant tells us, and polymerization reactions are not exempt from our classifying urges. So we classify these polymerization reactions.
We aren't going to list these classifications just yet.
But just coming up with a few categories isn't enough for us. We have to come up with different classification systems. And the different systems can often be confused. So we're going to try to make it clear as possible by listing the different systems right up front. The first system is the Addition-Condensation system.
This system divides polymerizations into two kinds, and if you're really clever you've probably figured out that those two kinds of polymerization are
Now, we call a polymerization an addition polymerization if the entire monomer molecule becomes part of the polymer. We call a polymerization a condensation polymerization if part of the monomer molecule is kicked out when the monomer becomes part of the polymer. The part that gets kicked out is usually a small molecule like water, or HCl gas. Such small molecules condense on things as tiny droplets, hence the name. Let's look at some examples to illustrate the point.
When ethylene is polymerized to make polyethylene, then every atom of the ethylene molecule becomes part of the polymer. The monomer is added to the polymer outright. You might say that an addition polymer is like a good friend who accepts
everything about you, the pleasant and the unpleasant alike.
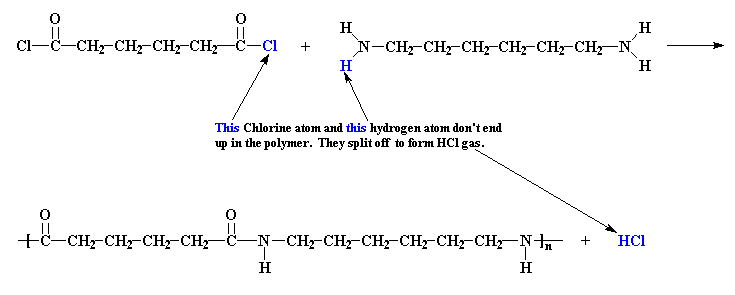
But a condensation polymer is more like a snotty social club that says, "Sure you can join, but only if you ditch those friends of yours". You see, in a condensation polymerization, some atoms of the monomer don't end up in the polymer. When nylon
6,6 is made from adipoyl chloride and hexamethylene diamine, the chlorine atoms from the adipoyl chloride, each along with one of the amine hydrogen atoms, are expelled in the form of HCl gas.
Because there is less mass in the polymer than in the original monomers, we say that the polymer is condensed with regard to the monomers. The byproduct, whether it's HCl gas, water, or whatnot, is called a condensate.
The Bottom Line
Want the short version? Condensation polymerizations give off byproducts. Addition polymerizations don't.
Now let's talk about the next system of classification: The Chain Growth - Step Growth system.
The other system of classifying polymerizations again divides polymerization reactions into two categories, and those are, all together now:
and
Very good. Now the differences between a chain growth polymerization and a step growth polymerization are a little more complicated than the differences between an addition polymerization and a condensation polymerization. It goes something like this:
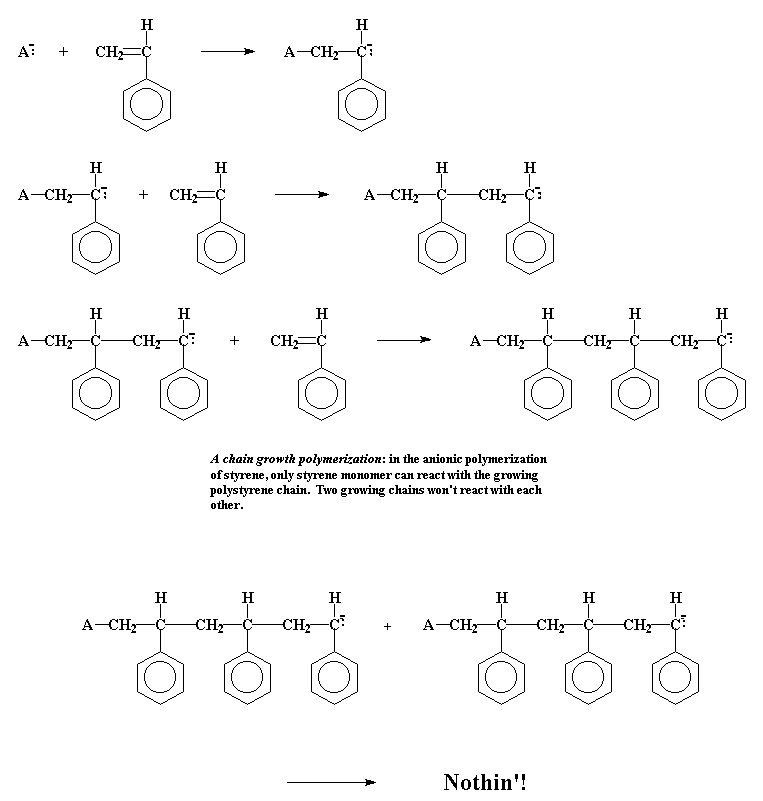
In a chain growth polymerization, monomers become part of the polymer one at a time. Pretty simple, huh? To show you how it works we've got a picture of a chain growth polymerization here, namely the anionic polymerization of styrene to make
polystyrene. Take a look at it.
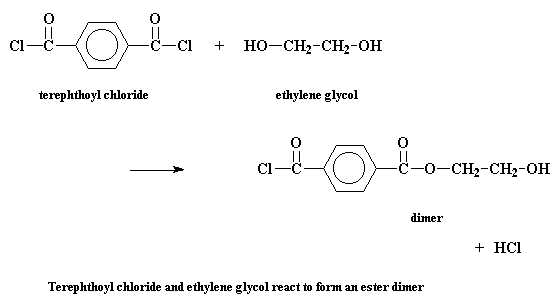
But in a step growth polymerization, things are more complicated. Let's take a look at the step growth polymerization of two monomers, terephthaloyl chloride and ethylene glycol, to make a polyester called poly(ethylene terephthalate). The first thing that happens is that the two monomers will react to form a dimer. Sounds simple enough.
Now at this point in a chain growth system, only one thing could happen: a third monomer would add to the dimer to form a trimer, then a fourth to form a tetramer, and so on. But here, in the free land of step growth polymerization, that dimer can do a lot of different things. It can of course react with one of the monomers to form a trimer:
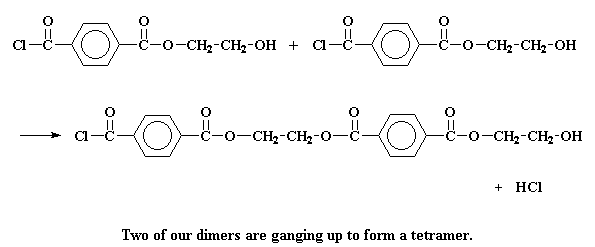
But it can do other things, too. It may react with another dimer to form a tetramer:
Or it may even get really crazy and react with a trimer to form a pentamer.
Making things more complicated, these tetramers and pentamers can react to form even bigger oligomers. And so they grow and grow until eventually the oligomers are big enough to be called polymers.
Just think of the way your bank keeps merging with other banks and getting bigger and bigger, and you'll get the idea.
The Bottom Line
The main difference is this: In a step growth reaction, the growing chains may react with each other to form even longer chains. This applies to chains of all lengths. The monmomer or dimer may react in just the same way as a chain hundreds of monomer units long. But in an addition polymerization, only monomers react with growing chains. Two growing chains can't join together the way they can in a step growth polymerization.
Now I'm sure a lot of you bright people out there noticed that our step growth polymerization to make a polyester produced a byproduct, HCl gas. This of course will make it a condensation polymerization as well as a step growth polymerization.
You may have also noticed that our chain growth polymerization of styrene did not produce a byproduct. Yes, this is an addition polymerization in addition to being a chain growth polymerization.
It's easy to conclude here that a step-growth polymerization and condensation polymerization are the same thing, and that a chain growth polymerization and an addition polymerization are the same thing. But this just isn't always true. There are addition polymerizations that
are step growth polymerizations. One example is the polymerization that produces polyurethanes. There are also condensation polymerizations that are chain growth polymerizations. Trying to reconcile the chain growth-step growth classification system and the addition-condensation classification system is really a waste of time. Each has its own criteria, and the distinctions made by one system are not always going to be the same as the distinctions made by the other.
So don't try to reconcile the two systems. Just know that polymerizations can be step growth or chain growth, and they can be condensation or addition.
The Addition-Condensation System
Addition Polymerization
Condensation Polymerization
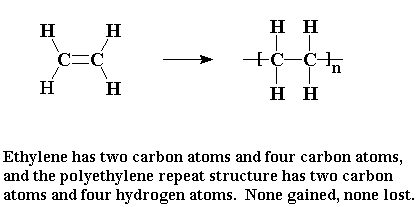
The Chain Growth - Step Growth System
Chain Growth Polymerizations
Step Growth Polymerizations
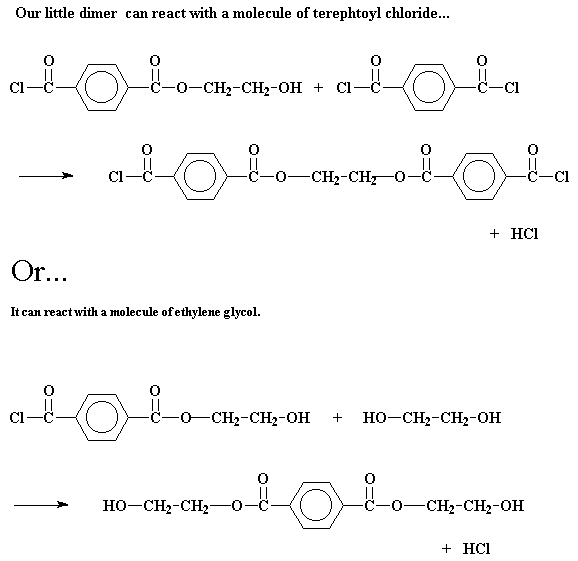
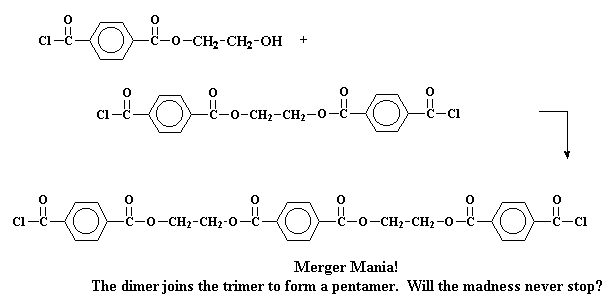
Irreconcilable Differences

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
