Flames in Space: Teacher's Notes
Activity: Effects of Gravity on the Burning of a Candle
From NASA
Microgravity: A Teacher's Guide with Activities, Secondary Level, Activity 7, p. 35-37.
Microgravity Science and Applications Division: Office of Space Science and Applications
Education Division: Office of Human Resources and Education
National Aeronautics and Space Administration
Publication EP-280, July 1992
Safety: First and Foremost
- If you choose to do any of the testing as a demonstration or as hands-on activities, it's essential to follow all safety precautions to the utmost. Students may need to be reminded that burns are painful and can be disfiguring, and trying any of these experiments (especially the cellulose nitrate, even as commercial flash paper sold in magic stores) on a larger scale can be fatal. It is our hope that in providing video footage of the more dangerous demonstrations, the need for individuals to do these in person will be nil, and thus students will be able to see the chemistry and learn from it without any risk whatsoever.
DISCLAIMER:
We offer this site as an educational tool. WE ARE NOT RESPONSIBLE FOR ANY INJURY OR DAMAGE CAUSED TO ANY PERSON, DIRECTLY OR INDIRECTLY, RELATING TO ANY OF THE DEMOS OR EXPERIMENTS LISTED AT THIS SITE. YOU ARE WHOLLY RESPONSIBLE FOR YOUR SAFETY.
Full Disclaimer
|
- Wear safety goggles at all times.
- Provide a safety shield for protection of the students and of you.
- Perform all experiments and demonstrations in a well-ventilated area, in open, still air. (That is, if you do this outdoors, a breezy day can be unpredictable and hazardous.)
- Never ignite anything in a sealed or closed container.
Objectives
- To intoduce the students to the observations and critical thinking
- To illustrate the effects of gravity on the burning rate of candles
National Science Education Standards: Content Standards
This activity fulfills the following within the Content Standards: 9-12
- Content Standard A: Science as Inquiry
Safety must be an integral component of their investigations.
- Content Standard B: Physical Science
- Structure and Properties of Matter
- Chemical Reactions
Materials
Birthday candles (several)
Matches
Balance beam scale (0.1 gm or greater sensitivity)
Clock with second-hand or stopwatch
Wire cutter/pliers
Wire
Small pan to collect dripping wax
Method
-
Form candle holders from the wire as shown in the diagram. Determine and record the weight of each candle and its holder.
-
Light the "upright" candle and permit it to burn for one minute. As it burns, record the colors, size, and shape of the candle flame.
-
Weigh the candle and holder and calculate how much mass was lost.
-
Place the inverted candle on a small pan to collect dripping wax. (Note: The candle should be inverted to an angle of about 70 degrees from the horizontal. If the candle is too steep, dripping wax will extinguish the flame.)
-
Light the candle and permit it to burn for one minute. As it burns, record the colors, size, and shape of the candle flame.
-
Weigh the candle and holder and calculate how much mass was lost.
Questions:
-
Which candle burned faster? Why?
-
How were the colors and flame shapes and sizes different?
-
Why did one candle drip and the other not?
-
Which candle was easier to blow out?
-
What do you think would happen if you burned a candle horizontally?
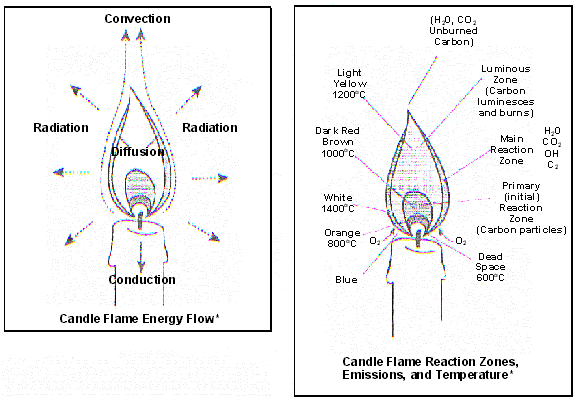
Candle flame diagrams adapted from "The Science of Flames poster," National Energy Foundation, Salt Lake City, UT.
For Further Research:
-
Burn a horizontally-held candle. As it burns, record the colors, size, and shape of the candle flame. Weigh the candle and calculate how much mass was lost after one minute.
-
Repeat the above experiments with the candles inside a large jar. Let the candles burn to completion. Record the time it takes each candle to burn. Determine how and why the burning rate changed.
-
Burn two candles which are close together. Record the burning rate and weight the candles. Is it faster or slower than each candle alone? Why?
-
Obtain a copy of Michael Faraday's book, The Chemical History of a Candle, and do the experiments described.
References
Faraday, M., (1988), The Chemical History of a Candle, Chicago Review Press.
Cornia, R., (1991), "The Science of Flames," The Science Teacher, v58n8, p. 43-45.
Other articles that might be of interest:
-
"A new use for the candle and tumbler myth," by Gavin D. Peckham, J. Chem. Educ. ,1993 70 1008.
-
"Demonstration of the burning of a candle," by Isidor S. Hirschhorn, J. Chem. Educ. ,1941, 18, 107.
-
"Evolution of the candle," by G. Griffin Lewis, J. Chem. Educ. , 1934, 11, 367.





