
Polyacrylate Basics
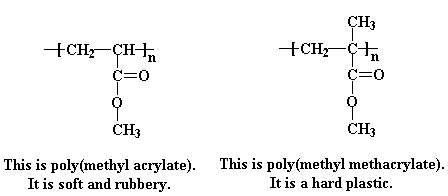
Keywords
gel
Acrylates are a family of polymers, which are a type of vinyl polymer. Acrylates are of course made from acrylate monomers, and it's about time we explained what those are. Acrylate monomers are usually esters which contain vinyl groups, that is, two carbon atoms double-bonded to each other, directly attached to the carbonyl carbon of the ester group. Some polymers (especially copolymers) contain the free acid, acrylic acid as shown below.
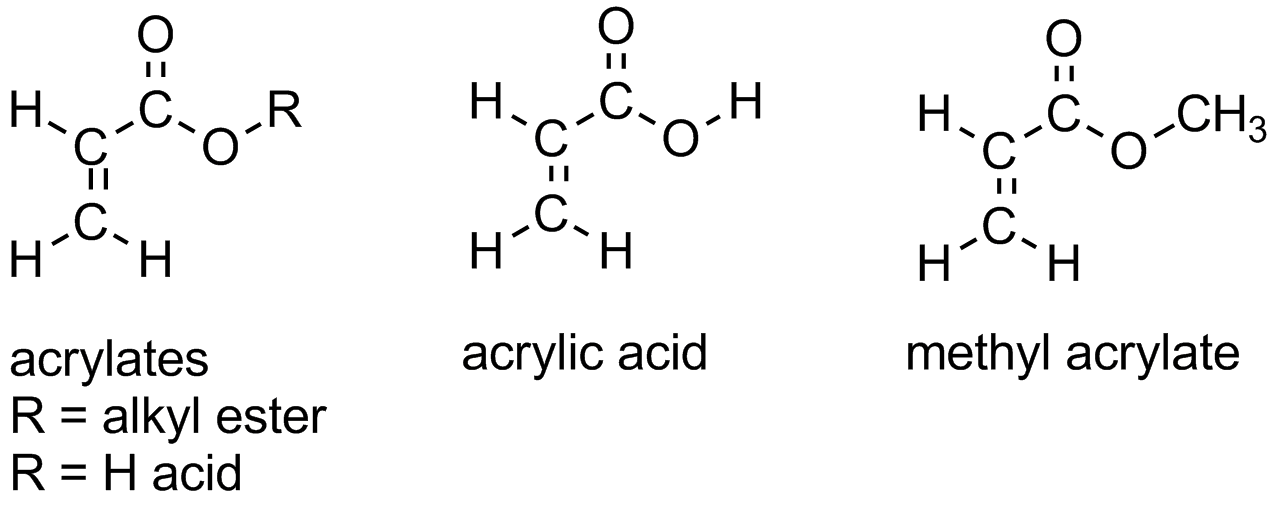
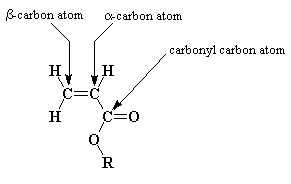
It's important to note the difference between the two vinyl carbons (the alpha and beta carbons to the ester or acid group as shown below).
Because of the very polar nature of the carbonyl, pulling electron density away from the normally electron-rich vinyl group,
the alpha carbon is more electron poor than the beta carbon. This has a huge effect on the reactivity of the monomer, something
we don't have space to go into here. One thing it means, though, is that anionic polymerization becomes possible for acrylates
(and methacrylates as well), and this gives polymers with very different backbone tacticities and very different
physical properties such as being more crystalline.
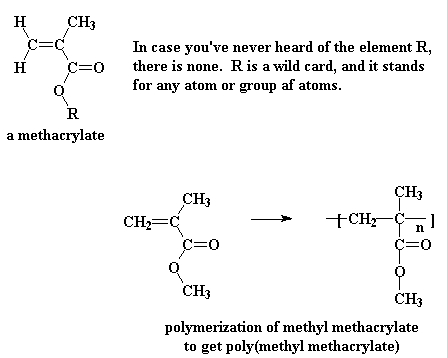
Some acrylates have an extra methyl group attached to the alpha carbon, and these are called methacrylates. One of the most common methacrylate polymers is poly(methyl methacrylate).
Acrylate and Methacrylate
one of Nietzsche's lesser known works
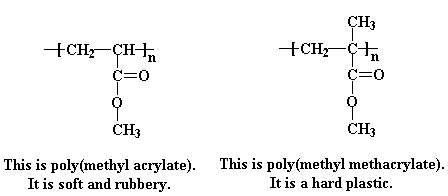
As it turns out, how soft or hard a polymer is at a given temperature is determined by what we call chain mobility, that is, how well the polymer chains wiggle past and around each other. The more they can move, the softer the polymer is. It helps here to think the scene in the movie Raiders of the Lost Ark, in which our hero Indiana Jones finds himself in an Egyptian temple and ankle deep in slithery poisonous snakes. Polymer chains are like those snakes. Smooth snakes can move past each other very easily. But if you could imagine those snakes having giant barbed spikes all up and down their backs like some of the dinosaurs in another Steven Spielberg film, you can see that they might not be moving around quite as much on the floor of that Egyptian temple. Their spikes would catch on each other, and slithering would become quite difficult.
Poly(methyl methacrylate) is like one of those snakes with giant barbed spikes all up and down its back, with those extra methyl groups acting like the spikes to put a quick stop to any slithering the poly(methyl methacrylate) chains would try to do. Poly(methyl acrylate), on the other hand, is like the smooth snakes. Without that extra methyl group getting in the way, they can slither all they want. If the polymer chains can slither and wiggle past and around each other easily, the whole mass of them will be able to flow more easily. So, a polymer which can move around easily will be soft, and one which can't will be hard, to put it simply.
So you're probably asking yourself that age-old question, "If a little is good, a lot should be much better, right?" Meaning, of course, if adding a simple methyl group to the vinyl carbon next to the ester had such an effect on polyacrylate properties, why not use even bigger groups? Great idea! Doesn't work. And here's why: sure, a bigger group would modify the polymer properties even more, but you just can't make any polymer. Anything bigger than a methyl stops the polymerization in its tracks. Steric hindrance becomes too great for the incoming monomer to add on to the end of the reactive polymer chain (which there isn't any of anyway). Kind of like that scene in an animated movie where a T-Rex is trying to eat this kid trapped in a corner: his head's just too big to fit in and take a bite. Ok, bad analogy, but you get the point, I hope.
If you want to know more about how polymers are like snakes take a look at the glass transition temperature page.
No More Saggy Diapers That Leak
The simplest acrylate polymer is one of the least well understood. And that would be...
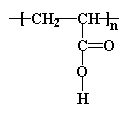
This is what we call a polyelectrolyte. That is to say, each repeat unit has an ionizable group. In this case, it's a carboxylic acid group. Poly(acrylic acid) and related acrylates are unusual because they soak up water like crazy. They absorb many times their own weight in water with no problem, even hundreds of times more. That means that even as little as a few grams of the polymer can soak up a cup of liquid and hold onto it tightly. Polymers that do this are called superabsorbers. So some bright individual had the bright idea of putting this stuff into baby diapers. In a diaper, poly(acrylic acid) absorbs all the liquid your little bundle of joy leaves behind. Remember all those diaper commercials where they pour that blue stuff on the diapers and it disappears? What you saw was poly(acrylic acid) or a similar acrylate polymer in the act of superabsorption.
And don't think for one minute that we completely understand why poly(acrylic acid) can absorb so much water, but it's clearly related to its molecular structure and properties.
The advantage of having diapers with poly(acrylic acid) in them, aside from the fact that they're less messy, is that once the mess is locked up in the poly(acrylic acid), baby doesn't have to sit in it until mom and dad figure out that it's time for a change. Otherwise, baby could get some unpleasant skin rashes.
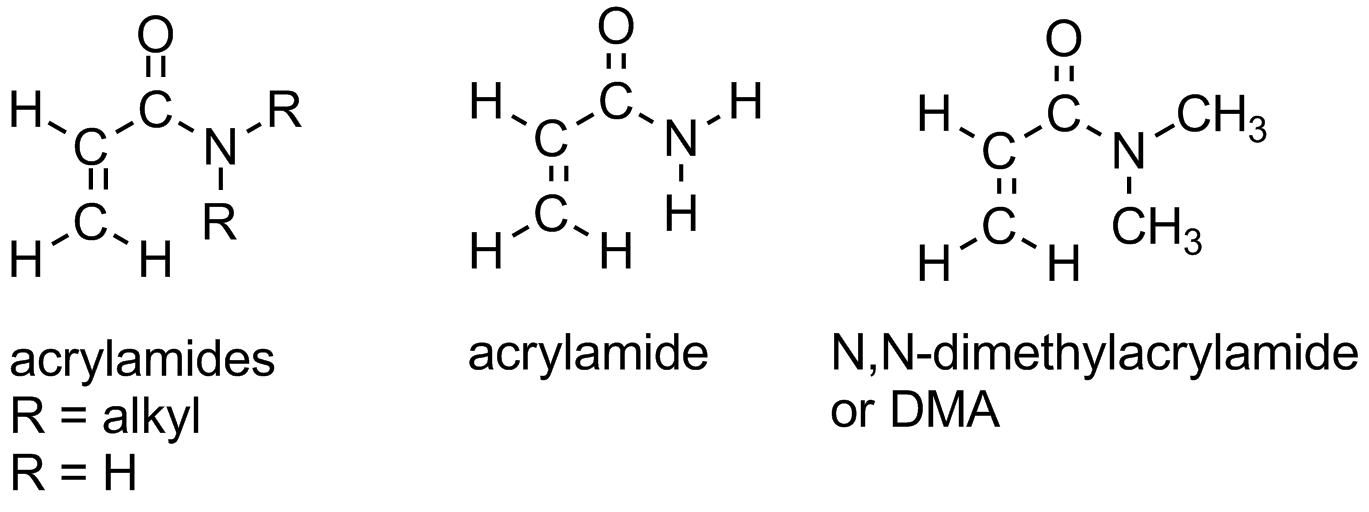
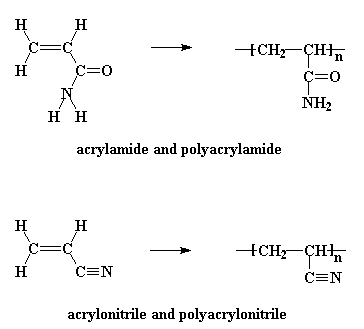
There are several derivatives of polyacrylates which contain nitrogen. Polyacrylamide and polyacrylonitrile are two shown in the picture. Polyacrylonitrile is used to make acrylic fibers. Polyacrylamide will dissolve in water and is used industrially in many applications needing this ability. Even crosslinked polyacrylamides can absorb water. Crosslinked polymers can't really dissolve, if you think about it, but that doesn't stop water from wanting to interact with it. These gels of water-swollen crosslinked polymer are used to make things like soft contact lenses. It's the absorbed water in them that makes them soft, but you need other comonomers or polymers mixed in with them to help with things like oxygen permeability. To see the polymer and its monomer acrylamide in 3-D, click here.
Making Acrylates More Useful
If you look at an acrylate, you'll see that it has two functional groups. One is the carboxylic acid group which allows making all kinds of derivatives using almost any alcohol or amine you can think of. The other functional group is the double bond, of course, which is what lets the acrylate undergo various kinds of polymerization. What if you had a third functional group present, something you could do additional reactions with, either on the monomer before polymerization or on that specific group after the polymer is formed?
Turns out this has been done lots using the ester group. Imagine an ester alkyl with an alcohol, amine, acid of various kinds or even another double bond in it somewhere. A simple example is the monoacrylate of ethylene glycol. It's an acrylate for sure, but the other end of the ester alkyl is an unreacted alcohol. When you have the methyl group on the double bond (of a methacrylate ester), then you have hydroxyethyl methacrylate, also known as HEMA; see the structure below for the monomer and polymer. The polymer has useful properties, being reactive and liking to bond with lots of other polar functional groups. That makes the polymer useful in biological systems for a number of applications that we don't have time to go into here, but hey, you can always do a web search to find out more!
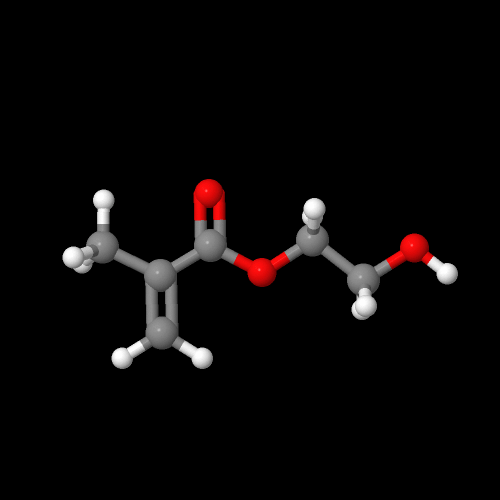
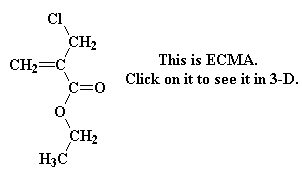
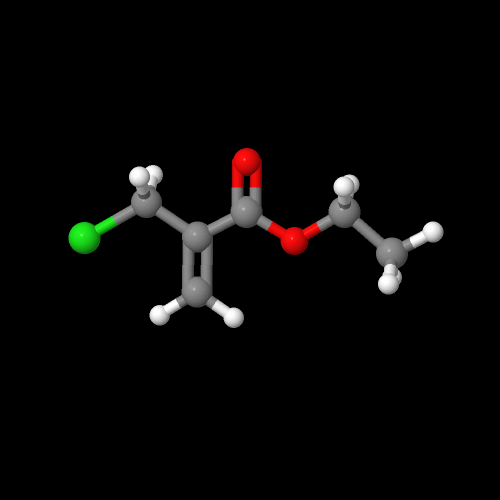
It turns out there's a simple way to make more complex acrylate monomers and polymers: use a functional group on the methyl of methacrylates. The images below show some examples, made from the alpha-hydroxymethyl monomer. And it turns out that the chloromethyl group of ECMA allows you to make all kinds of functionalized acrylate polymers.
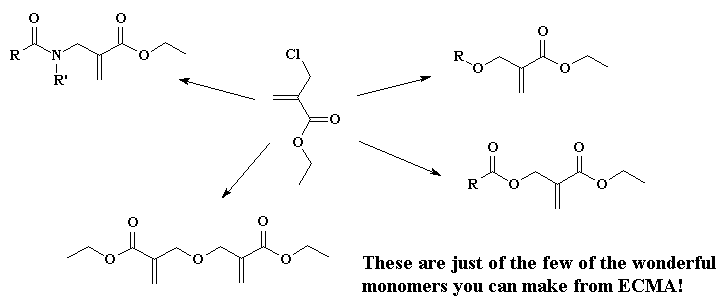
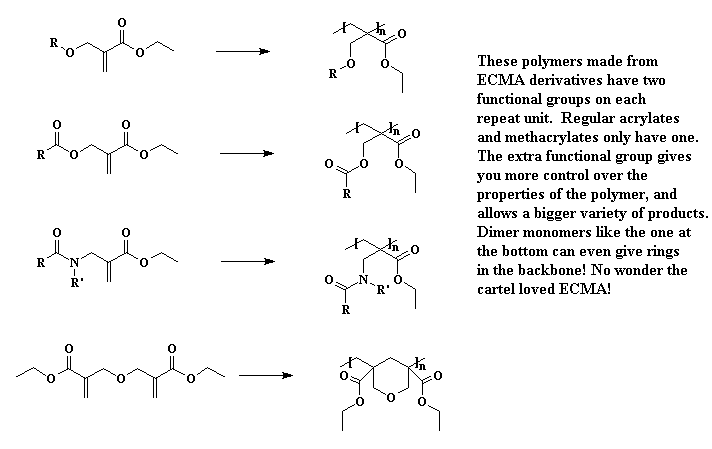
Detailed Synthetic Procedures
What's that you say? You'd actually like to make some of these fascinating polymers? Well, hold your horses (as if you have any), cause here are some recipes and detailed procedures. Just click on the one you're interested in or click on the second link to download the pdf file. Be careful and have fun!
For free radical polymerization of methyl methacrylate to give (mostly) atactic PMMA, click here to view the procedure and here to download a copy.
For anionic polymerization of methyl methacrylate to give (mostly) syndiotactic polymer, click here to view the procedure and here to download a copy.
For the general procedure to make RHMA monomers with various ester groups (methyl, ethyl, butyl and t-butyl) and conditions for free radical polymerization to give corresponding homopolymers, click here to view the procedure and here to download a copy.
And next is the general procedure to make RHMA ether-linked dimers with various ester groups (methyl, ethyl, butyl and t-butyl) and conditions for free radical cyclopolymerization to give soluble cyclopolymers, view here and here to download a copy.
Now for free radical polymerization of ethacrylic acid, a normally difficult to make polymer because of the alpha-substituent bigger than methyl; click here to view the procedure and here to download a copy.
For anionic polymerization of isopropyl acrylate to give (mostly) isotactic polymer, click here to view the procedure and here to download a copy.
And for anionic polymerization of isopropyl acrylate to give (mostly) syndiotactic polymer, click here to view the procedure and here to download a copy.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |