 |
Ultra Violet Curable coatings use a UV light to initiate crosslinking. There are no solvents in UV curables, rather oligomers, photoinitiators, reactive diluents (used for crosslinking), and fillers or additives. The coating that is applied is a viscous liquid, this is due to the reactive diluent and the oligomers units. Once the coating has been applied to a particular substrate it is flashed with UV light. The process for cure is either a free radical or cationic mechanism and once fully cured the coating is a high molecular weight, crosslinked, and non-tacky film. The structures below represents the components of a UV curable system. All of the components in the UV curable system react and form the polymer coating network. Therefore 100 percent of the applied coating remains on the substrate once the coating is cured. No VOCs are emitted after cure and the coating is termed environmetnally friendly.
 |
| Component | Percent | Function |
| Photoinitiator | 1-3 | Free radical or cationic initiation |
| Reactive diluents | 15-60 | Film formation and viscosity control |
| Oligomer polymer | 25-90 | Film formation and basic properties |
| Additives and fillers | 1-50 | Surfactants, pigments, stabilizers, etc. |
| Lamp Source | Energy input (cure) |
Below are structures of Initiators:
Free radical
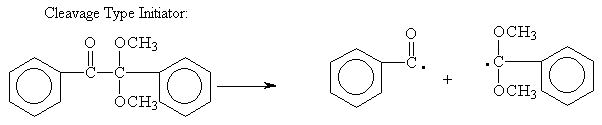
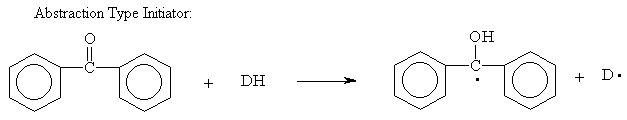
Cationic
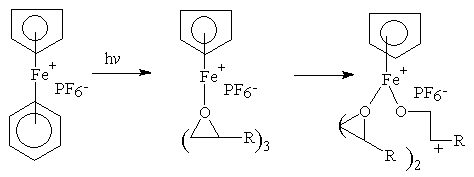
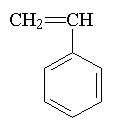
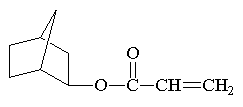
Here are some of the common
monomers used in a UV curable system.
|
|
|
|
 |
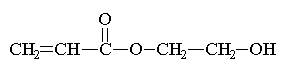 |
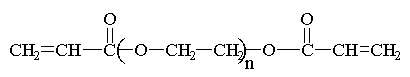 |
|
|
|
|
 |
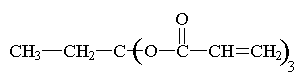 |
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Some of the established applications for UV curable coatings are: printings inks, metal, textile, platics, wood, and paper coatings, vinyl floors, fiberglass lamenates, photoresists fir printed circuits and microeletronics, photoresists for photochemical machining, moisture vaporbarrier coatings, and photopolymers for printing plates and photolithography.
![]() return to the Environmentally Friendly
Coatings page
return to the Environmentally Friendly
Coatings page