 |
Interpenetrating Polymer Networks, or more easily said IPNs, are a very interesting area of polymers. IPNs are basically two or more polymers, that don't want to be together, stuck together by a polymer scientist's dirty tricks. These two or more polymers are trapped together and are continous throughout the polymer network. IPNs have found there way into coatings, mainly in the form of Latex IPNs (LIPNs). LIPNs have been used in platic modifications, coatings, adhesives, damping materials, high polymer for medical applications, and ion exchange resins. This page will focus on LIPNs for coatings.
The technique
used to sythesize,or make, these LIPNs is mainly emulsion
polymerization. The product of an emulsion polymerization is
termed a latex, thus the name Latex INPs. How do they use emulsion
polymerization to make these LIPNs, good question. First let us talk
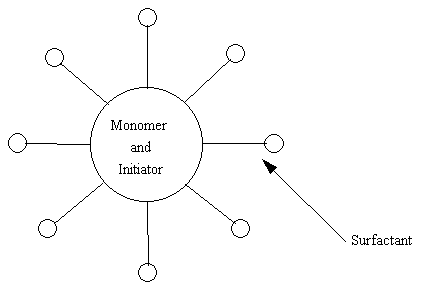
just briefly about the process of emulsion polymerization. To produce
an emulsion polymer you need a few things: water, surfactant (or
soap), and monomers and initiators. The whole process is done in
water, which is considered the continuous phase. The monomers used
in the emulsion polymer usually don't mix with water so the surfactant
is used to emulsify or mix the monomers in the water. You can think
about it this way. When you do the dishes and clean a greasy frying
pan the grease forms little droplets in the water, but when you had the
"grease cutting" soap those droplets disappear and the grease leaves the
pan. What happen is the soap likes both the water and the grease
so is lets the two mix. Emulsion polymerization uses the surfactant
the same way, it allows the monomers to mix with the water and then the
reaction can take place. As you can see below the surfactant surrounds
the monomers and initiators and allows them to be dispersed in the water.
 |
Latex IPNs are synthesized in a similar fashion, but with
a little twist. In a standard emulsion polymerization the monomers
are feed into the water and surfactant over time. To make a Latex
IPN you feed in your first monomer, let it react completely, then you feed
in you second monomer along with a difunctional monomer, and let them react
completely. This process is referred to as staging and creates a
new structure, or morphology. The crosslinking agent in the second
stage The morphology for these particular Latex IPNs are called core-shell.
Where the core and the shell are completely different polymer structures.
The core could be a hard polymer and the shell a soft polymer, or vice
versa (hard and soft polymer refers to the glass transition
temperature of each polymer).
 |
On some ocassions the second monomer will not be compatable with the first polymer so a compatiblizer is added. A compatibilizer is something that is added to join the two polymer phases in the polymer particle. However, in coatings the first and second polymer are usually somewhat compatible.
This core shell morphology gives rise to different properties than a standard emulsion polymer. As a emulsion polymer coating, or waterborne coating, is applied the polymer particles in water become closer together as the water evaporates. Once these particles are close enough they begin to flow together, or coalesce. This coaelescence of the particles creates the IPN, two polymers, or phases that are traped together yet are continuous throughout the polymer network. Click here if you would like to learn more about IPNs.
References:
D.J. Hourston, R. Satgurunathan, H. Varma. Journal of Applied
Polymer Science. 215(1987).
Z. Liucheng, L. Xiucui, L. Tianchang. Journal of Applied Polymer
Science. 891(1991).
![]() return to the Main page
return to the Main page