There are many types of polymer systems that are used in coatings. One type of polymer system is the polyester system. Polyesters are synthesized from polyfunctional carboxylic acids and alcohols.
|
|
| Wide ranges of monomers for unlimited properties and substrates |
| Excellent cost / performance |
| Good Adhesion |
| Good mechanical properties |
Polyester synthesis for coatings
can be done by the following methods:
| REACTANTS | REACTION CONDITIONS | REACTION BYPRODUCTS |
| Direct polyesterification
Dicarboxylic acid + glycol Hydroxycarboxylic acids |
High temperature in bulk or solvent
acid catalysts, metal oxides or salts |
Water |
| Ester exchange
Dialkyl diester + diol Diaryl diester + diol |
High temperature in bulk
base catalysis |
Alcohol
Phenol |
| Transacylation
Diacid + diacetate of a Bisphenol |
High temperature in bulk
base catalysis |
Alknoic acid |
| Acylation
Di-COCl + bisphenol |
High temperature in bulk or inert
solvent; low temperature with tert- amines as catalysts |
HCl |
| Acylation
Di-COCl + bisphenoxide |
Low temperature interfacial
condensation with acceptor for HCl |
Cl- |
The end use of the polyester coating
will determine which polyfunctional carboxylic acids and polyfunctional
alcohols are used in the polymer synthesis. Some polyacids and polyols
impart flexibility and toughness, some give stain resistance and chemical
resistance. The monomers of choice depend on the function of the
final coating. Here are some of the polyacids and polyols used in
polyester synthesis and the properties they each bring to the party.
| Polyacids: | |
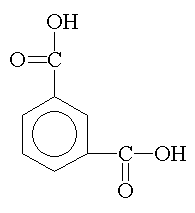 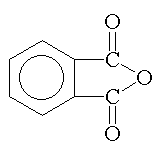
isophthalic anhydride phthalic acid |
Phthalic anhydride and isophthalic acid are used extensively because of their ease of handling, good balance of properties, and economy. Isophthalic acid is preferred over phthalic anhydride when a tougher, more flexible, faster drying, and more chemically resistant coating is desired. |
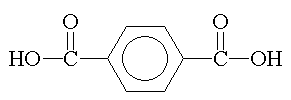
terephthalic acid |
Replacing phthalic anhydride or isophthalic acid with terephthalic acid increases crystallinity. |
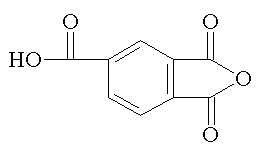
trimellitic anhydride |
Trimellitic anhydride and pyromellitic dianhydride introduce branching to the polymer and contribute to the aromaticity of the polymer. |
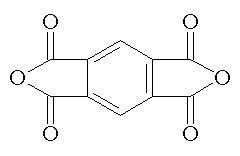
pyromellitic dianhydride |
Trimellitc anhydride and pyromellitic dianhydride provide carboxyl groups useful for producing water dispersible polymers. |
| Polyols: | |
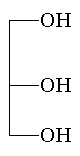
glycerol |
Glycerol is widely used in oil-modified polyesters but because of its tertiary hydrogen is replaced by trimethylol ethane or trimethylol propane for high performance polymers. |
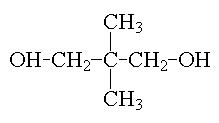
2,2,4-trimethyl-1,3-pentanediol |
The bulky, asymmetrical structure of 2,2,4-trimethyl-1,3-pentanediol provides for low polymer viscosity; also the steric shielding of the ester linkages and better hydrophobicity contribute to excellent corrosion resistance, stain resistance, and detergent resistance. |
Neopentyl glycol |
Neopentyl glycol has no beta hydrogens and therefore imparts high chemical resistance and stability to heat, hydrolysis, and weathering. The compact molecular structure of neopentyl glycol allows close packing of polymer chains, resulting in excellent stain resistance. |
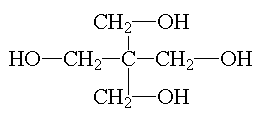
pentaerythritol |
Pentaerythritol and dipentaerythritol are used in the synthesis of oil-modified polyester resins for industrial coatings. They allow for crosslinking because of the number of functional sites eachpolyol has. |
dipentaerythritol |
These are just a few of the polyacids and polyols that are used for the synthesis of polyesters coatings. Any polyfunctional acid or alcohol can be used and mixtures of polyols or polyacids is also utilized. The sky is the limit to the combinations of polyols and polyacids used.
References:
Handbook of Polymer Synthesis, Part A, Hans Kricheldorf, ed., p. 647,
1992.