

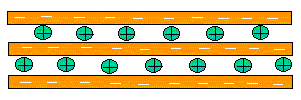
For these reasons, the clay must be treated before it can be used to make a nanocomposite. After all, these stacks of clay platelets are much larger than one nanometer in every dimension. Making a composite out of untreated clay would not be a very effective use of material, because most of the clay would be stuck inside, unable to interact with the matrix.
A popular and relatively easy method of modifying the clay surface, making it more compatible with an organic matrix, is ion exchanging. The cations are not strongly bound to the clay surface, so small molecule cations can replace the cations present on the clay. For example, in the picture below, the green cations are sodium ions. Some of them have been replaced by another cation.
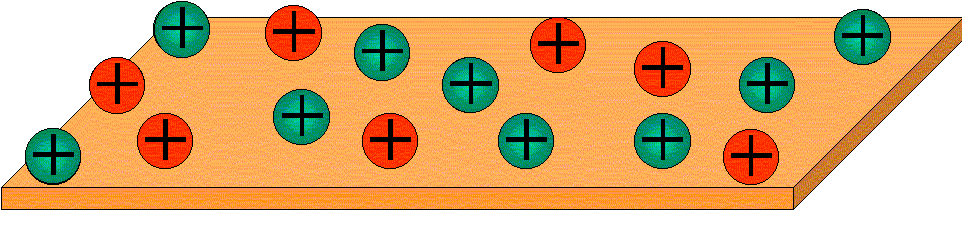

If the red cations are quaternary ammonium ions with long alkyl chains, this clay would be much more compatible with an organic matrix. By exchanging it with various organic cations, montmorillonite clay can be compatibilized with a wide variety of matrix polymers. At the same time, this process helps to separate the clay platelets so that they can be more easily intercalated and exfoliated. Nanocomposites can then be made from the intercalated or exfoliated clay. An ion exchanging process is used in the production of the first commercial clay nanocomposite.