
Nonlinear Polymer Structures
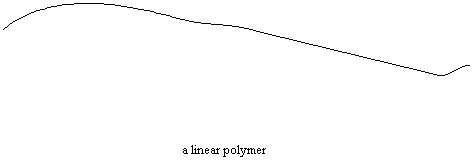
Many polymers are built so their molecules consist of many thousands of atoms arranged into long linear chains. But they don't have to be long straight chains. Polymers can be made in a lot of other arrangements, too. Let's look at a few of them, shall we?
Branching Out
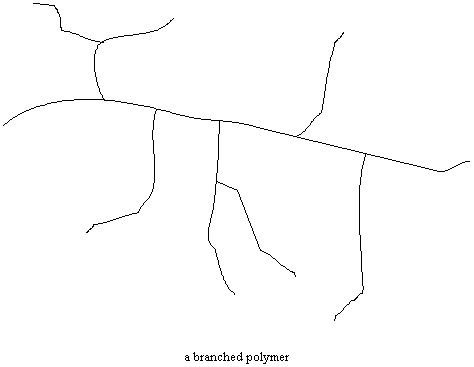
Not all polymers are linear like in the figure above. Sometimes there are chains attached to the backbone chain which are comparable in length to that backbone chain. This is called a branched
polymer. Some polymers, like polyethylene, can be made in linear or branched versions. As you might guess, the different molecular structures lead to materials with very different properties.
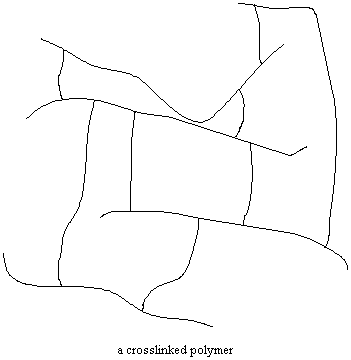
Network is Not a Verb
The branch chains have some strange habits. Sometimes, both ends of the branch chains are attached to the backbone chains of separate polymer molecules. If enough branch chains are attached to two polymer molecules, it can happen that all of the polymer backbone chains in a sample will be attached to each other in one giant network! When this happens, the sample is in fact one single molecule, a molecule large enough to pick up in your hands! Polymers like this are called crosslinked polymers. Many types of rubber, such as polyisoprene and polybutadiene, are crosslinked.
A tire is actually one giant network molecule, a molecule so big it takes
two hands to pick it up. A bowling ball contains only one molecule, too. Remember those old fashioned black rotary telephones? You got it, the housing is made of one molecule!
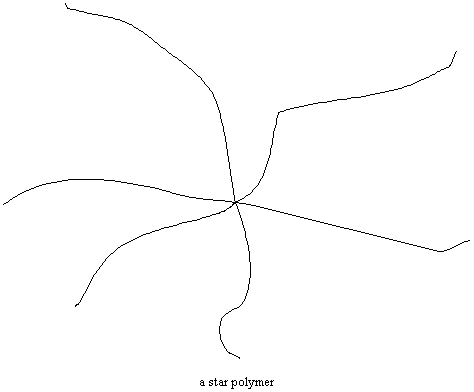
Starlight, Starbright…
Sometimes the ends of several polymer chains are joined together at a common center. Polymers like this are called star polymers. They're used as additives in motor oil.
Plant a Tree. It's Good For the Earth.
Sometimes there is no backbone chain at all. Sometimes a polymer
is built in such a way that branches just keep growing out of
branches and more branches grow out of those branches. These are
called dendrimers, from the ancient Greek word for "tree".
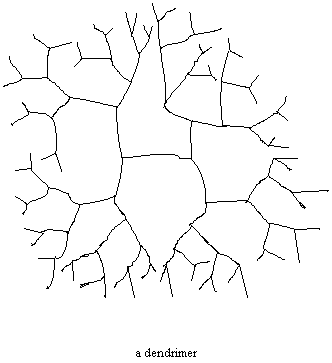
Dendrimers have unusual shapes that make pretty pictures, and they also have really unusual properties. Drug delivery is one possible use for dendrimers. One silicon-based dendrimer can trap oxygen molecules in its branches. It is hoped that this can be someday used to make artificial blood. In the more immediate future, dendrimers might end up in coatings and/or as catalysts.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |