
Basic chemistry of polymers
You may have started your visit here knowing nothing about polymers, so I can understand if some of you feel lost in this topic. This page will give you a brief introduction to help you understand a little more about polymers. We'll concentrate on the basic chemistry although you should know there are lots of other aspects of polymer science involving processing, property evaluation, engineering and applications.
It turns out that most polymers, both natural and synthetic, have mainly carbon, hydrogen, oxygen, and nitrogen as their most common elements. DNA and RNA also have phophorous, but we'll leave that for a later discussion.
If you don't know anything about chemistry, than you can learn some here, and trust me when I say that carbon, hydrogen, oxygen and nitrogen are among the most common elements found in nature on this planet. That's probably why they show up in so many natural polymers. It's also the reason they're used in so many synthetic polymers. Being readily available means they're also pretty inexpensive. More importantly, they have properties that make them ideal for constructing complicated polymer molecules with useful properties.
Now it turns out that these four elements can bond to themselves and to each other. That bonding generates molecules. For example water, H2O, is made up of two hydrogen atoms and one oxygen atom. They're bonded together to make the water which is so necessary for life on this planet. By the way, just because our seas and oceans are full of water, that doesn't mean we can waste it or pollute it by throwing garbage in. We only have one planet- good old Earth- and if we mess up the most important natural resource we have (yeah, water), well, you can figure that one out yourself.

Lets talk about Carbon
Carbon is number 6 in the periodic table, and is the basic building block for all the polymers that you will find in these web pages.
Did you know that you are a carbon being? You eat food made mostly of carbon. Your proteins and genes contain carbon. Even the fat that most of us wish we didn't have is made mainly of carbon. Your body uses the energy stored in your food by converting it to useful chemicals (made of carbon) and the host of polymers (again, mostly carbon) that make up your skin, hair, muscles and blood. In short, without carbon, you wouldn't be.
'C' is the abbreviation for carbon, and it can bond with practically anything. Carbon always has four bonds to other atoms, although sometimes it makes more than one bond to something else. A one carbon molecule attached to four hydrogen atoms is called "methane," which I'm pretty sure you've heard of as a major part of natural gas. The molecule methane looks like this:
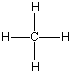
Now I said that Carbon can bond with most anything and does, but it always likes to keep all four bonds attached to something else. So if it is bonded to something, like another carbon atom, than the other three bonds will be connected to something else, which is usually hydrogen but could also be a combination of oxygen, nitrogen and more carbon atoms. Below are four molecules. Starting with methane I have attached a carbon to each of the base atom's four bonds, one by one.
Each of these molecules is real, found in oil and petroleum refining. Each is perfectly happy and stable as it's drawn here. But wait! There's more!
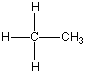 | 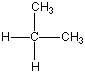 | 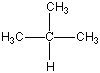 | 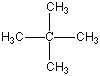 |
Carbon can also have multiple bonds. It can bond with itself, oxygen, and nitrogen using more than one bond to one or more of these atoms. Multiple bonds between two carbon atoms look like this:
As I said before, carbon is the basis for a lot of what you will see in these pages. It is found in two main forms; long chains like this:
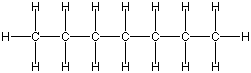
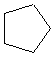
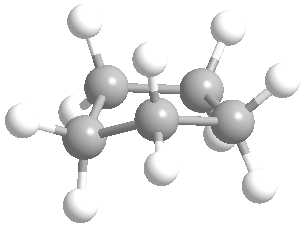
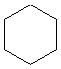
Or cyclic rings like the ones below. A cyclic ring with six carbons having all single bonds is called cyclohexane. It is usually shown schematically as the structure the left but a more accurate depiction of the bonds and angles is in the middle and on the right:
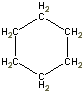
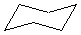
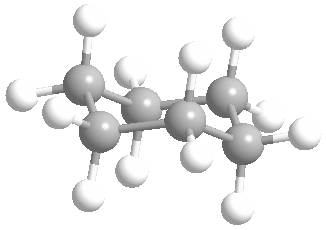
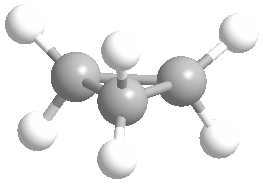
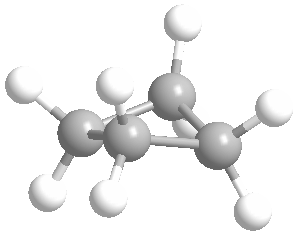
Cyclic rings can be found with as little as three carbons to many, many more, but most often they have only a few atoms with six carbons being the most stable. They are also drawn without the carbons on every corner, so you just have to know that where two lines come together like this, there's a carbon there. The way these are drawn, it is just assumed that the entire ring is made up of carbon atoms with enough hydrogens on each to make up four bonds.
Now if you look at the images to the right of each of the rings, you'll see an actual 3-dimensional depiction of that ring. All the carbons and hydrogens are shown, and the angles and bond lengths are pretty much what they are at the molecular level.
 |
 |
 |
 |
 |
 |
 |
 |
Pretty cool, huh? They look like those blocks we used to have so much fun shoving into the holes of a red plastic box when we were wee tots, remember? These rings can stand by themselves as perfectly happy molecules, or they can be part of larger molecules. They can even be joined together in long polymer chains- crazy, huh?
Both of these structures for cyclohexane are correct, but the farthest right one is called the chair conformation to show something about how the ring actually looks in 3-dimensional space, but not as well as the actuall 3D structure on the right of each ring. Don't worry, you won't be tested on this stuff- at least not yet.
Poly-what?
Now we can begin to talk about polymers. Polymers are long chains of linear or cyclic structures. A monomer unit, for example, may only have a propylene structure with two carbons in the chain and one pendent to it, but that structure is then repeated many times over. This makes each chain or molecule very long and very large. The result is called a polymer or macromolecule. The polymer molecule we see below contains one of the simplest repeat units (3 carbons, remember?) linked together with bonds between carbons of adjacent units.
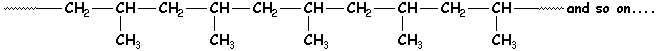
The dotted lines at the end of this molecule indicates that it
continues on, with many other atoms attached that you just can't see here.
So in other words, the real picture would be very much larger than what we have
room for on this page.
And let's not forget that nature makes polymers, too. In fact, natural polymers were around long before humans even figured out what a polymer was, like millions of years before! A good example of a natural polymer that is a major part of trees and cotton is cellulose:

Polymers are everywhere around and in us. They are in things that we eat, items that we use, and are crucial parts of all living creatures. Basically, there is no getting away from polymers. That is why it is so important for us to understand them and how to use that knowledge to improve what we have and do.
So that's enough to get you started on the chemistry of polymers. But to learn more about the polymers around you, visit the Macrogalleria or the Kid's Macrogalleria. There is also a link, below, to a list of other sites with periodic tables in case you'd like to see different ways all the atoms of our universe are organized.
Links to sites with periodic tables
Return to the Infocenter
Return to the Polyquarium