Polymers from Microorganisms
Plankton, eaten by baleen
whales, the
whale shark, and some
crustaceans such as clams, is a very
good example of tiny organisms which are
beneficial. True, they are not technically polymers, but they have
lots of polymers in their tiny bodies. Some of those polymers are
crucial to how those tiny structures form and behave.
Foraminifera and radiolarians are very tiny
creatures called microorganisms. They exist in huge numbers in the seas
and when they die and sink to the bottom, they form deposits of
chalk and limestone that we see in various places around the world. Think about
the chalk teachers used to have for their blackboards. The reason
these creatures do this is because of what they are made of.
 Calcium carbonate is a common inorganic substance
found in these organisms that does not decompose easily. Therefore when
the organisms die they leave calcium carbonate behind, which in large
numbers (millions of millions) can create deposits. A good example of
chalk deposits would be the White Cliffs of Dover.
Calcium carbonate is a common inorganic substance
found in these organisms that does not decompose easily. Therefore when
the organisms die they leave calcium carbonate behind, which in large
numbers (millions of millions) can create deposits. A good example of
chalk deposits would be the White Cliffs of Dover.
 Deposits are then mined for the valuable materials they hold.
Products made with calcium carbonate added include cement,
plastics, and poly(vinyl chloride).
Plastic coffee cups like the one on the left are
examples of polymers containing calcium carbonate.
Deposits are then mined for the valuable materials they hold.
Products made with calcium carbonate added include cement,
plastics, and poly(vinyl chloride).
Plastic coffee cups like the one on the left are
examples of polymers containing calcium carbonate.
Because of its low cost calcium carbonate is also used to improve paper,
a product of cellulose from trees, and is used to
control sheen or gloss in flat paints. It is also used as a base in
cosmetics and toothpastes to neutralize acids in the mouth and on the face.
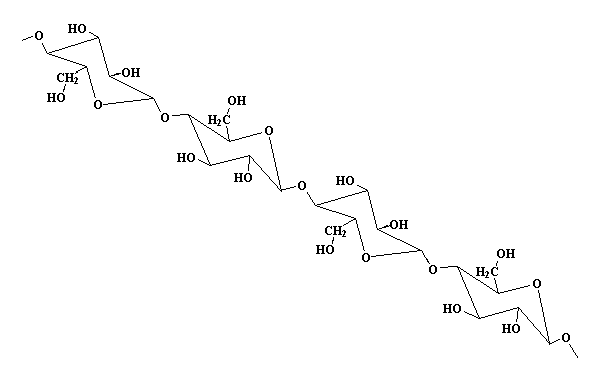
In case you were wondering, cellulose looks like this:
There are organisms that assist in cleaning up oil and other chemical spills. This process is called bioremediation. It has been a large help to both the ocean, its inhabitants, and humans who use the oceans resources in every day life.
There are some organisms that have been found in very extreme environments, such as heat as high as 350 degrees! Because of their tolerance for heat these organisms can be used in reactions which produce medicines. They can also be used in polymerase chain reaction, also known as chain growth polymerization which is used in DNA fingerprinting.
There are even tiny creatures
 that are so small and slow to work
that it just looks like rust, but actually are organisms slowing eating
away at the metal that have unfortunately found its way into their
habitat.
They have already gotten the name 'metal eating bacteria'. They
have caused much damage, not only to this airplane that went down into
the ocean, but also to industrial sites. Many companies are trying to
engineer building structures that are made from polymer materials
which can resist this metal eating bacteria.
that are so small and slow to work
that it just looks like rust, but actually are organisms slowing eating
away at the metal that have unfortunately found its way into their
habitat.
They have already gotten the name 'metal eating bacteria'. They
have caused much damage, not only to this airplane that went down into
the ocean, but also to industrial sites. Many companies are trying to
engineer building structures that are made from polymer materials
which can resist this metal eating bacteria.What about other metal things like fish hooks?
Good question! Let's stray from the organism point of view and comment on it. What polymer scientists are trying to do is solve problems like these by finding new polymers to coat metals to stop rust and corrosion. They're also looking for other polymeric materials that will dissolve even faster than metals rusting so that, if they are lost, such as when a fishing hook and fishing line get carried away by a too-big fish, they will quickly be absorbed into the environment without harm. Now THAT'S a good use of science, no?
Return to the Polyquarium