


The model on the right above is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
For polybutadiene at a glance, click here!
Polybutadiene was one of the first types of synthetic elastomer, or rubber, to be invented. It didn't take a great a degree of
imagination to come up with, as its very similar to natural rubber,
polyisoprene. It's good for uses which require exposure to low temperatures. Tires treads are often made of polybutadiene copolymers. Belts, hoses, gaskets and other automobile parts are made from polybutadiene, because it stands up to cold temperatures
better than other elastomers. Many polymers can become brittle at low temperatures thanks to a phenomenon called the glass
transition. Driving in the winter can be bad enough with out hoses and gaskets going out on you! A hard rubber called poly(styrene-butadiene-styrene), or SBS rubber is a copolymer
containing polybutadiene.

Polybutadiene is a diene polymer, that is, it's a polymer made from a monomer that contains two carbon-carbon double bonds, specifically butadiene. It is made by Ziegler-Natta polymerization.

This is what a molecule of the monomer butadiene looks like:
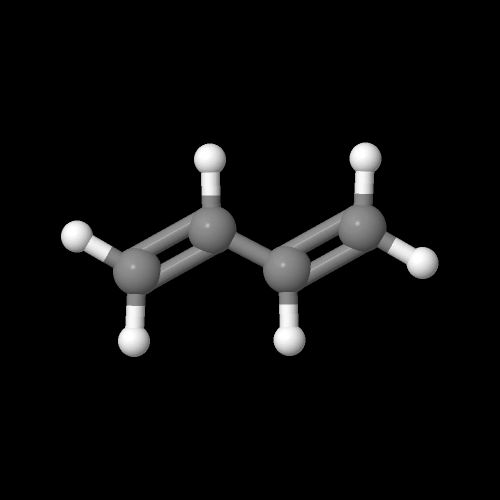
The model on the right above is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
Other polymers used as rubber include:









