

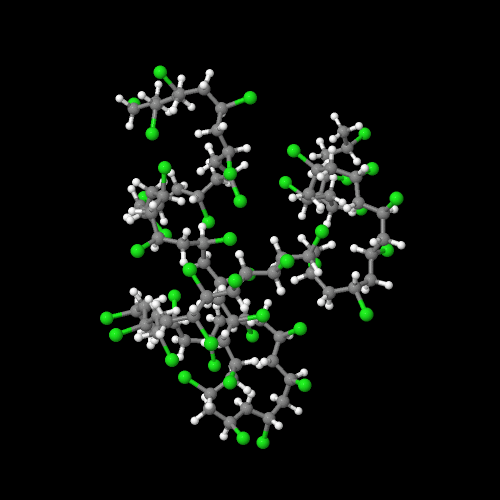
The model on the right above is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
PVC is useful because it resists two things that hate each other: fire and water. Because of its water resistance it's used to make raincoats and shower curtains, and of course, water pipes. It has flame resistance, too, because it contains chlorine. When you try to burn PVC, chlorine atoms are released, and chlorine atoms inhibit combustion.
Structurally, PVC is a vinyl polymer. (well, duh!) It's similar to polyethylene, but on every other carbon in the backbone chain, one of the hydrogen atoms is replaced with a chlorine atom. It's produced by the free radical polymerization of vinyl chloride.

And here, my friends, is that monomer, vinyl chloride:
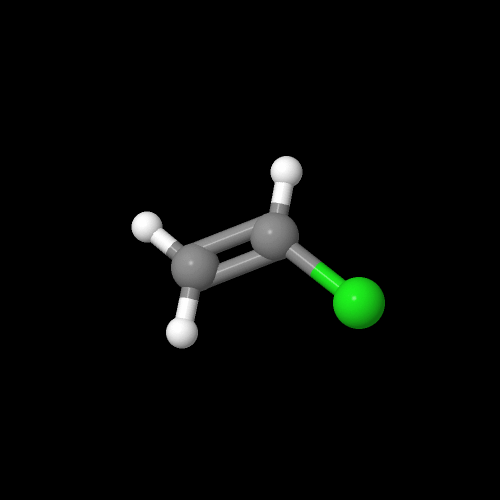
The model is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
PVC was one of those odd discoveries that actually had to be made twice. It seems around a hundred years ago, a few German entrepreneurs decided they were going to make loads of cash lighting people's homes with lamps fueled by acetylene gas. Wouldn't you know it, right about the time they had produced tons of acetylene
to sell to everyone who was going to buy their lamps, new efficient electric generators were developed which made the price of electric lighting drop so low that the acetylene lamp business was finished. That left a lot of acetylene laying around.
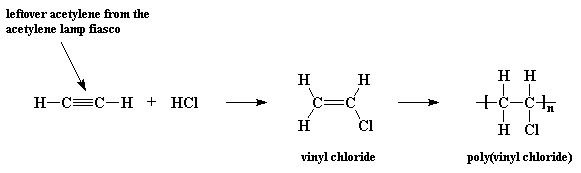
So in 1912 one German chemist, Fritz Klatte decided to try to do something with it, and reacted some acetylene with hydrochloric acid (HCl). Now, this reaction will produce vinyl chloride, but at that time no one knew what to do with it, so he put it on the shelf, where it polymerized over time. Not knowing what to do with the PVC he had just invented, he told his bosses at his company, Greisheim Electron, who had the material patented in Germany. They never figured out a use for PVC, and in 1925 their patent expired.
Wouldn't you know it, in 1926 the very next year, an American chemist, Waldo Semon was working at B.F. Goodrich when he independently invented PVC. But unlike the earlier chemists, it dawned on him that this new material would make a perfect shower curtain. He and his bosses at B.F. Goodrich patented PVC in the United States (Klatte's bosses apparently never filed for a patent outside Germany). Tons of new uses for this wonderful waterproof material followed, and PVC was a smash hit the second time around.
Other polymers used as plastics include:
Burke, James; Connections, Little, Brown and Co., Boston, 1978.
Fenichell, Stephen; Plastic: The Making of a Synthetic Century,
HarperCollins, New York, 1996.

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
