
Copolymer Concepts
Mixing Things Up
When a polymer is made by linking only one type of small molecule, or monomer, together, it is called a homopolymer. When two different types of monomers are joined in the same polymer chain, the polymer is called a copolymer. Let's imagine now two monomers, which we'll call A and B. A and B can be made into a copolymer in many different ways.
When the two monomers are arranged in an alternating fashion, the polymer is called, of course, an alternating copolymer (below). One interesting fact about this type is that the ratio of the two monomers is exactly 1:1. Very few copolymerizations give this type of structure, however.
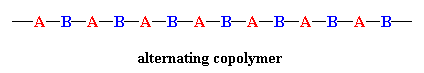
In a random copolymer, the two monomers may follow in any order (figure below). The ratio of the monomers incorporated into the copolymer is a result of a combination of the properties of the monomers, the polymerization conditions and the conversion of the polymerization, to name a few variables. For example, unless the two monomers have exactly the same reactivity, both with the other comonomer and with their own monomers, the ratio in the product will NOT be exactly 1-to-1. In fact, in most cases it's not, and this results in a change in the copolymer composition as the reaction proceeds. At the beginning, the more reactive monomer is incorporated more than the less reactive one.
But things change as monomers are used up and the concentration of the more reactive one decreases faster/more than that of the less reactive one. Things even out at some ratio of concentrations, giving polymer that is about 1-to-1 in composition. But now there's less of the more reactive one so it's used up faster as the reaction continues, causing the ratio of concentrations to change further till there's mostly just the less reactive monomer present. Copolymers made at this point will have more of the less reactive monomer. While you might measure an "average" composition of monomers in the final product (using NMR or FTIR or some other method), the composition of individual chains may (will) be much different than that average. And here's the kicker: the total combination of all those copolymer chains, varied in composition as they are, determines the final properties of the material made. So as they say nowadays: "It's complicated..."
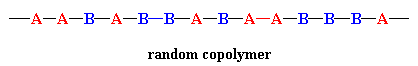
In a block copolymer, all of one type of monomer are incorporated together in one part of the chain, and then all of the other are reacted in somehow. A block copolymer can be thought of as two homopolymers joined together at one of the ends (below). Not surprisingly, it's not easy or cheap to make such a copolymer, so in fact, there aren't many commercial examples.
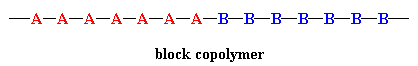
A blocky copolymer that you know very well, that is, if you wear shoes, is SBS rubber. It's used for the soles of shoes and for tire treads, too. "Blocky" means it has some of the characteristics of a true block copolymer but isn't as uniform in composition.
When chains of a polymer made of monomer B are grafted onto a polymer chain of monomer A we have a graft copolymer (see the figure). There are a number of ways to do this: grafting from; grafting to; or the most controlled way of using a "macromonomer." Say what? Yeah, sounds a little strange, but we're talking about a long polymer chain with a single functional group at the end that can react with the small comonomer molecules present to give the grafted structure.
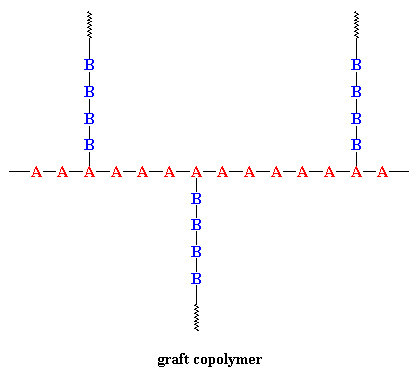
One kind of commercial graft copolymer is high-impact polystyrene, or HIPS for short. It's a polystyrene backbone with chains of polybutadiene grafted onto the backbone. The polystyrene gives the material strength, but the rubbery polybutadiene chains give it resilience to make it tough and less brittle.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |