At the very center of each atom is the nucleus (new-klee-us). The nucleus is a cluster of particles called protons and neutrons. Protons have a plus (+) charge, and neutrons are neutral (that is, they don't have a charge).
 The nucleus is very compact, and even though it accounts for most of an atom's weight, it takes up a very small amount of the atom's total volume. Weird, huh - it would be like if just about all of your weight was in your belly button. (but way smaller!)
The nucleus is very compact, and even though it accounts for most of an atom's weight, it takes up a very small amount of the atom's total volume. Weird, huh - it would be like if just about all of your weight was in your belly button. (but way smaller!)
As a matter of fact, most of the atom is empty space! The rest of the atom has electrons whizzing around it like crazy. Electrons have a minus (-) charge, and so there are enough electrons to balance out the (+) protons. Electrons are so light, though, that they really don't count towards the total weight of the atom.
But electrons don't fly around just anywhere. They have set amounts of energy. The more energy an electron has, the further away from the nucleus it tends to be. The outermost electrons are called the valence electrons (sounds like "vay-lents"). Valence electrons have a special job - they can form bonds - or connect - with another atom.
Atoms can have as many as 8 valence electrons. (Hydrogen and helium, those little troublemakers, can have 2, but no more.) A carbon atom has 4 valence electrons.
The kinds of atoms that exist are called elements. Some elements are: silver, gold, neon, and carbon.
To see all of the elements in one place, look at a periodic table. All of the elements that exist are there on the periodic table, starting with hydrogen at number 1, on up to more than 100.
That number, called the atomic number tells how many protons each atom has. So, hydrogen has 1 proton, carbon has 6 protons, and nickel has 28 protons.
Every element has a symbol of one or two letters. Some of them make sense, like carbon is C and oxygen is O, and some of them don't, like the symbol for gold, Au. (Actually, Au is based on the Latin word for gold, aurum.)
When atoms join together, they form bonds.
A Bonding Experience
There are two basic kinds of bonds - covalent and ionic.
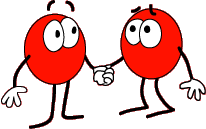
Covalent bonds happen when two atoms share electrons - kind of like 2 atoms holding hands. When at least 2 atoms get together by sharing electrons, they form a molecule.
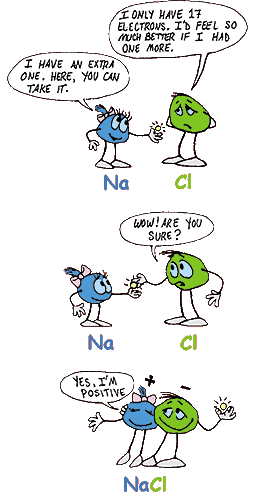 Ionic bonds happen when one atom gives at least one electron to another atom. Awwww, isn't that nice?!
Ionic bonds happen when one atom gives at least one electron to another atom. Awwww, isn't that nice?!
Picture this: Two atoms sit next to each other. One atom needs an electron, and the other atom has an extra electron. Perfect! Once the electron gets handed over, the atoms are no longer atoms - they're ions, and they each have a charge - one plus (+ positive) and one minus (- negative).
Remember that each atom started with enough (-) electrons to match each (+) proton in its nucleus. The atom that gets an extra electron ends up with a (-) charge and is called an anion (sounds like ann-eye-on). The atom that gives away an electron ends up with a (+) charge and is called a cation (sounds like cat-eye-on).
Now, those (+) and (-) charges have a strong attraction to each other - they sit next to each other and refuse to move. And guess what? That's an ionic bond! - the strong attraction between ions with opposite charges. Table salt is a good example of a common ionic compound. (Table salt is also called sodium chloride.) Click here for even more info.See a 3-D model of the ions here.
What about polymers? How are they bonded together?
Polymer backbones are held together by covalent bonds - by atoms sharing electrons. Other atoms, or even groups of atoms, hook onto the backbone by covalent bonds too.
Some polymers that are called ionic polymers have ionic pendant groups. Their backbones are still held together by covalent bonds.

Just in case you wondered...
Sometimes you'll see a drawing of an atom with a great big nucleus with rings around it, showing the electrons orbiting the nucleus like the planets around the sun. That's a simple way of seeing an atom, but it's not really correct. Reality is much more complicated (and harder to draw)!
|

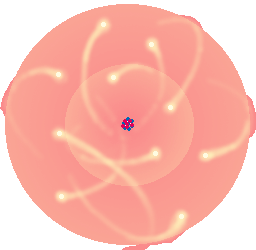
 The nucleus is very compact, and even though it accounts for most of an atom's weight, it takes up a very small amount of the atom's total volume. Weird, huh - it would be like if just about all of your weight was in your belly button. (but way smaller!)
The nucleus is very compact, and even though it accounts for most of an atom's weight, it takes up a very small amount of the atom's total volume. Weird, huh - it would be like if just about all of your weight was in your belly button. (but way smaller!)
 Ionic bonds happen when one atom gives at least one electron to another atom. Awwww, isn't that nice?!
Ionic bonds happen when one atom gives at least one electron to another atom. Awwww, isn't that nice?!

