
|
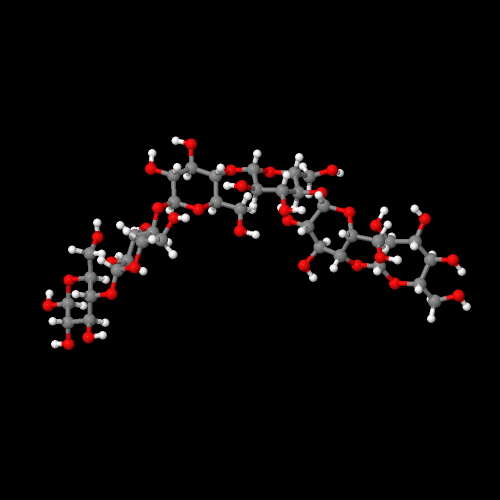 Click the picture to see a 3-d interactive version of cellulose. |
Made by Nature
All kinds of plants - like carrots, apple trees, and flowers - make the polymer CELLULOSE (sell-you-low-s). Plants use cellulose to make their stems and branches and leaves strong.
Cellulose makes tree trunks strong enough to hold up the tallest trees! We can even build tall houses out of wood. Wood is also used to make paper. Did you know that paper is mostly cellulose?
Cotton is Mostly Cellulose!
The cotton-ball part of the cotton plant has a lot of cellulose.
 Cellulose makes great fibers. Thanks to cellulose, cotton fibers can be twisted into thread and woven into cloth. Cotton fibers are even found in some fancy kinds of paper, and that makes the paper feel more cloth-like.
Cellulose makes great fibers. Thanks to cellulose, cotton fibers can be twisted into thread and woven into cloth. Cotton fibers are even found in some fancy kinds of paper, and that makes the paper feel more cloth-like.
Why is cellulose so strong?
Why does it make great fibers?
Let's find out by looking at what the polymer chains are like.
| Cellulose chains are kind of like raw spaghetti: all stretched out and lined up next to each other. Imagine these sticks of spaghetti much, much longer, and stuck to each other, too. |

|
|
A Closer Look at Cellulose
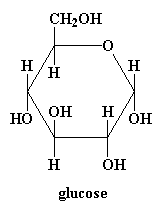
Plants make cellulose from glucose, a simple sugar. Plants know how to connect the glucose monomers in a way that lets the chain stretch out, nice and straight.
Monomer: Glucose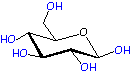
|
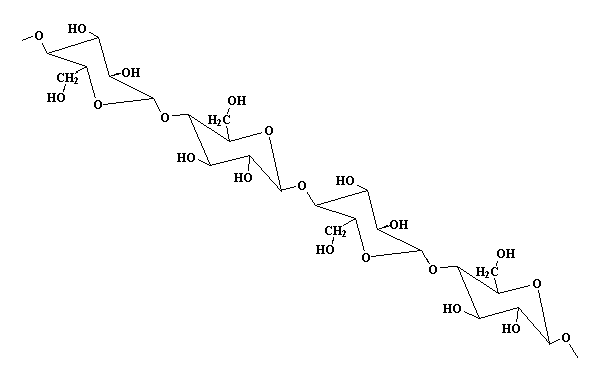
Polymer: Cellulose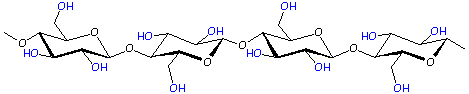
We left the C atoms (and most of the H's) out of this drawing to make it look nicer.
Click here to learn more about drawing molecules like this. |
When the chains lie next to each other, there are -OH groups in-between the chains. See them in blue? There are three -OH groups attached to each ring, and they can act like little magnets for each other. All these little magnets
 The science term for this awesome sticky force (between chains of cellulose) is called hydrogen bonding. The "H" acts like the (+) end of a magnet, and the "O" acts like the (-) end. When they're close, they stick. If it was just a few hydrogen bonds, they could pull apart (like pulling apart two weak magnets). But when you add up a whole bunch of them on a long polymer chain, they make the material very stiff and strong!
The science term for this awesome sticky force (between chains of cellulose) is called hydrogen bonding. The "H" acts like the (+) end of a magnet, and the "O" acts like the (-) end. When they're close, they stick. If it was just a few hydrogen bonds, they could pull apart (like pulling apart two weak magnets). But when you add up a whole bunch of them on a long polymer chain, they make the material very stiff and strong!
Click on the image below to see one way that cellulose chains can form hydrogen bonds.

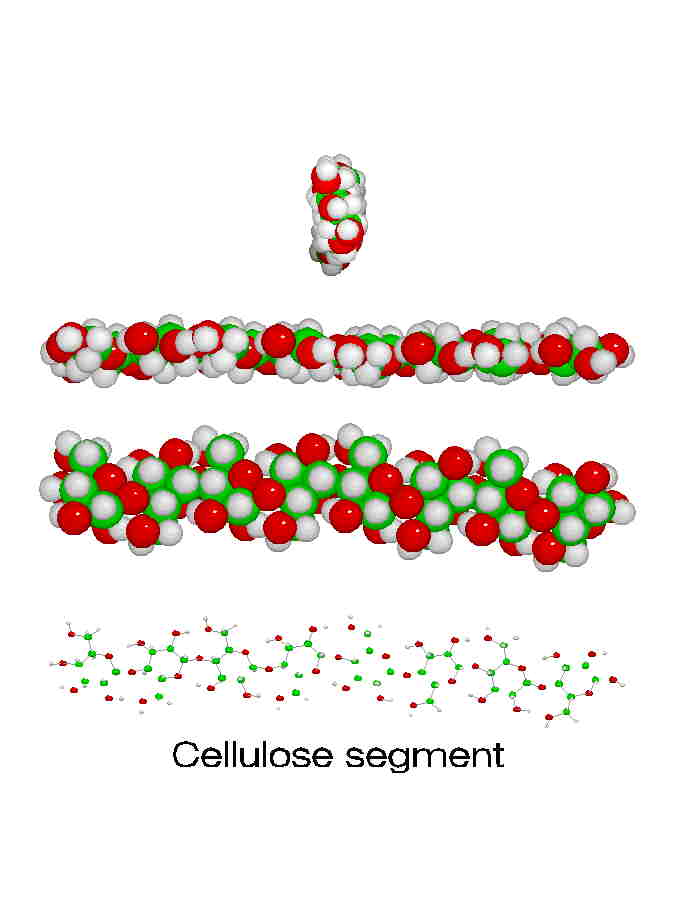 Wood, paper, and cotton all contain cellulose. Cellulose makes excellent fibers. One way to see why this is involves looking at the molecular structure. Notice how straight and regular its shape is, especially in the 3-D model above. That linear shape means the molecules can all pack tightly next to each other with hydrogen bonds between each chain and its neighbors. Think of all the strands of string that make up a rope: they all line up in the same direction so that when you pull on the rope, you're pulling on every strand in that rope tugging against its neighbors. As they saying goes, "Strength in numbers!" but only if they all work together...
Wood, paper, and cotton all contain cellulose. Cellulose makes excellent fibers. One way to see why this is involves looking at the molecular structure. Notice how straight and regular its shape is, especially in the 3-D model above. That linear shape means the molecules can all pack tightly next to each other with hydrogen bonds between each chain and its neighbors. Think of all the strands of string that make up a rope: they all line up in the same direction so that when you pull on the rope, you're pulling on every strand in that rope tugging against its neighbors. As they saying goes, "Strength in numbers!" but only if they all work together...
Here are four views of celluose chains on the right, from side-on and edge-on to end-on. These should help you see why they can pack together so well to make such strong fibers.
Cellulose is made of repeat units of the monomer glucose - the same monomer found in regular sugar. Because cellulose is built out of a sugar monomer, it is called a polysaccharide. This is the same glucose which your body uses in order to live, but you can't digest it in the form of cellulose. Still, it is an important part of your diet. The cellulose fibers from vegetables and grains help scrub out your intestines and keep them clean. Click here for more about fiber in your diet.
More about Glucose

Glucose is used by plants and animals for energy and to build structural parts of plants. There's one problem - we can't digest cellulose! So eating trees (or grass or straw doesn't help us much at all. The good news is that plants can connect glucose monomers together in a different way, so that they twist all around and form a big globby polymer called starch. (Potatoes and rice contain a lot of starch!)
Your body can digest the starch back down into glucose molecules, and then use the glucose for energy to run and jump and play and think! Click here to learn more about starch. You've heard of carbos or carbs? These are short-hand words for carbohydrates that are sugars and starches we can digest.
Now take a look at glucose in the diagram below. Click on the figure and see it in 3-D! Notice any difference between the pop up and what's on this page? Right! They're isomers with the one OH on the ring next to the ether (ring oxygen) either up or down. The one on this page is the alpha isomer with the OH down while the one in the pop up is the beta isomer. Alpha is used in starch, beta in cellulose. Amazing how much of a difference the isomer makes in physical properties of their polymers: mashed potatoes you can eat versus tree trunks you can't.

Both cellulose and starch are natural polymers. That means that some plant or animal made them, and in this case, plants. Click here to learn more about how starch and cellulose differ. There are other natural polymers made up of amino acids (proteins) and nucleic acids (RNA and DNA). To learn more about other polymers made by nature, click here.
Polymers Made From Cellulose

NOW PLAYING at the Macroplex Cinema! |
Not only is cellulose everywhere in nature, it was also used to make some of the first synthetic polymers like cellulose nitrate, cellulose acetate, and rayon. Forms of these polymers are still made and used today.
Cellulose nitrate - also called nitrous cellulose or nitrocellulose for short - was originally used to make plastics often used as imitation ivory. It was also used to make motion picture film. But it was highly explosive and caused a lot of fires in movie theaters. This stuff is still around in a much safer form which is still used for plastics and clear lacquer coating for furniture, musical instruments and other wood objects.
Cellulose acetate was what replaced cellulose nitrate in movie film. It is not explosive and is still used to make film negative, print film and clear plastic sheets. In the old days of movies it was often called celluloid, and this name is still used to refer to movie film, even though a lot of the release prints for movies are now made of more durable polyester. Cellulose acetate is also used to make fibers for acetate fabric.
Rayon was originally a fiber made from cellulose nitrate. But like the plastic and film versions it was also very flammable. The new rayon - made from cellulose xanthate - is much safer and less flammable than the old stuff. Rayon is pretty popular for fabric because it has a lot of the qualities of natural plant fibers. This makes sense, since that's what it is made from. But it also has a smooth texture that makes it shiny like silk. One of the first uses of rayon was as an inexpensive replacement for silk.
Other polymers used as fibers include: Polyethylene Polypropylene Polyester Polyacrylonitrile Polyurethanes Nylon

|
Return to Kinds of Polymers |

|
Return to Main Page |