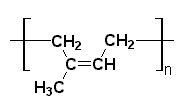
polyisoprene

On this page we're going to learn more about natural rubber. Natural rubber is a polymer called polyisoprene. Chemists draw it's chemical structure like this:

For those of you who are new to polymer chemistry, the brackets and the letter n mean that the structure drawn is repeated over and over again. Little units like the one in the picture above are joined together to form a long chain, like this:
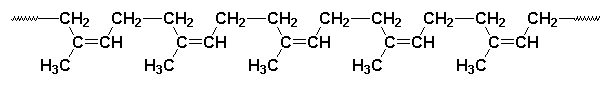
In natural rubber the chain has thousands of repeat units, not just five like you see in the picture above.
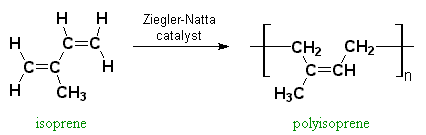
Polyisoprene can be made synthetically by polymerization of a small molecule called isoprene, with the help of special metal compounds called Ziegler-Natta catalysts.
As it comes from the tree, natural rubber latex is not good for very many things. The resourceful people of the Americas in pre-Columbian times used it to make waterproof boots, and balls for playing games like basketball. But the boots could only be used in warm climates. In cold weather they would become brittle and crack. So people in cold places could only use natural rubber for pencil erasers. That's where the word rubber comes from.