|
 Click the picture to see a 3-d interactive version of cellulose. |
All kinds of plants - like carrots, apple trees, and flowers - make the polymer CELLULOSE (sell-you-low-s). Plants use cellulose to make their stems and branches and leaves strong.
Cellulose makes tree trunks strong enough to hold up the tallest trees! We can even build tall houses out of wood. Wood is also used to make paper. Did you know that paper is mostly cellulose?
The cotton-ball part of the cotton plant has a lot of cellulose.
 Cellulose makes great fibers. Thanks to cellulose, cotton fibers can be twisted into thread and woven into cloth. Cotton fibers are even found in some fancy kinds of paper, and that makes the paper feel more cloth-like.
Cellulose makes great fibers. Thanks to cellulose, cotton fibers can be twisted into thread and woven into cloth. Cotton fibers are even found in some fancy kinds of paper, and that makes the paper feel more cloth-like.
Let's find out by looking at what the polymer chains are like.
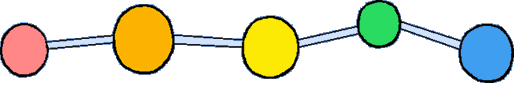
| Cellulose chains are kind of like raw spaghetti: all stretched out and lined up next to each other. Imagine these sticks of spaghetti much, much longer, and stuck to each other, too. |

|
Plants make cellulose from glucose, a simple sugar. Plants know how to connect the glucose monomers in a way that lets the chain stretch out, nice and straight.
Monomer: Glucose
|
Polymer: Cellulose
We left the C atoms (and most of the H's) out of this drawing to make it look nicer.
Click here to learn more about drawing molecules like this. |
When the chains lie next to each other, there are -OH groups in-between the chains. See them in blue? There are three -OH groups attached to each ring, and they can act like little magnets for each other. All these little magnets
 The science term for this awesome sticky force (between chains of cellulose) is called hydrogen bonding. The "H" acts like the (+) end of a magnet, and the "O" acts like the (-) end. When they're close, they stick. If it was just a few hydrogen bonds, they could pull apart (like pulling apart two weak magnets). But when you add up a whole bunch of them on a long polymer chain, they make the material very stiff and strong!
The science term for this awesome sticky force (between chains of cellulose) is called hydrogen bonding. The "H" acts like the (+) end of a magnet, and the "O" acts like the (-) end. When they're close, they stick. If it was just a few hydrogen bonds, they could pull apart (like pulling apart two weak magnets). But when you add up a whole bunch of them on a long polymer chain, they make the material very stiff and strong!
Click on the image below to see one way that cellulose chains can form hydrogen bonds.

 Glucose is used by plants and animals for energy. There's one problem - we can't digest cellulose! Plants can connect glucose monomers together in a different way, so that they twist all around and form a big globby polymer called starch. (Potatoes make a lot of starch!)
Glucose is used by plants and animals for energy. There's one problem - we can't digest cellulose! Plants can connect glucose monomers together in a different way, so that they twist all around and form a big globby polymer called starch. (Potatoes make a lot of starch!)
Your body can digest the starch back down into glucose molecules, and then use the glucose for energy to run and jump and play and think! You've heard of carbos? Carbos (short for carbohydrates) are sugars and starches. Click here to learn more about starch.
Click here to learn more about how starch and cellulose differ.
Even though we can't digest cellulose, it's still an important part of your diet. The cellulose fibers from vegetables and grains help to scrub out your intestines and keep them clean. Click here for more about fiber in your diet.
To learn more about other polymers made by nature, click here.
Other polymers that are used as fibers include:

|
Return to Kinds of Polymers |

|
Return to Main Page |