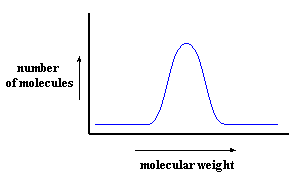
One way to get a plot like this is to use size exclusion chromatography, or SEC. But SEC doesn't really measure molecular weight. It measures hydrodynamic volume, that is, how big the coiled up polymer chain is in solution. This gives a relative measure of molecular weight, because obviously, the higher a polymer's molecular weight, the greater its hydrodynamic volume.
But sometimes you need to know exactly what the molecular weight distribution is. So it was left to some clever scientists to invent something we call matrix-assisted laser desorption/ionization mass spectroscopy, or MALDI mass spec for short. Sometimes we're really lazy and we just call it MALDI.
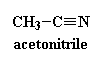 So what is this fantastic thing called MALDI? To explain, we'll walk
through the MALDI process to see just how it takes place. First of all,
we take our polymer, and we dissolve it in a solvent. What kind of
solvent? Well that all depends. The first MALDI experiments were done on
proteins. Now proteins tend to be soluble in
water, so water was the primary solvent. It was common to use 70:30
mixture of water and acetonitrile (click on it to see it in 3-D). The
solvent you use really depends on what kind of solvent will dissolve the
polymer you are studying.
So what is this fantastic thing called MALDI? To explain, we'll walk
through the MALDI process to see just how it takes place. First of all,
we take our polymer, and we dissolve it in a solvent. What kind of
solvent? Well that all depends. The first MALDI experiments were done on
proteins. Now proteins tend to be soluble in
water, so water was the primary solvent. It was common to use 70:30
mixture of water and acetonitrile (click on it to see it in 3-D). The
solvent you use really depends on what kind of solvent will dissolve the
polymer you are studying.
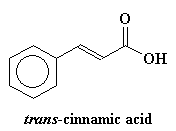
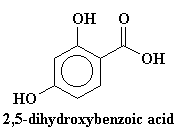
We also add a special ingredient. The special ingredient is a compound like trans-cinnamic acid or 2,5-dihydroxybenzoic acid. It varies from polymer to polymer, but the important thing is that our special ingredient has to absorb ultraviolet light. Usually we put about 104 times more of our UV absorber than polymer.
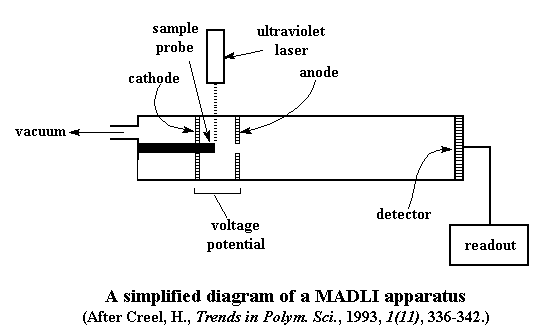
Did someone say laser? That's right, at this point we get to shoot a laser at our sample. That may not sound too exciting to you younger folk out there, but for us older people who grew up before lasers were ubiquitous it seems pretty amazing. We usually use an ultraviolet laser in the 330-360 nm range. Remember our sample is just loaded with UV-absorbing material. It loves UV light, and can't get enough of it. So it absorbs all the energy it can from the laser. Of course, it also passes some of that energy along to the polymer molecules.
The matrix material also reacts with the polymers in such a way that the polymers become charged ions. No one really knows how this happens. The fact that the polymers are now ionic will be very important in just a moment.
But first let's get back to all that energy that the polymers are absorbing from the matrix material. when they absorb all this energy some of the polymer molecules do something polymers almost never do. They evaporate. Usually polymer molecules are way too big and heavy to evaporate, but at these high temperatures and low pressures, they can do it. That's where the word desorption comes from in the name.
Now that we've got our polymers floating around in gaseous state, it's time I told you something important about this chamber. At the end of the chamber where we're evaporating our polymer molecules we've got two electrodes, a positive cathode, and a negative anode. Depending on what kind of polymer and what kind of matrix material you use, the polymers may be cations or anions. For this explanation we'll say the polymer we're looking at forms positive cations.
Now when we vaporize our polymer, we vaporize it right between the two electrodes. When our polymer forms cations, we place the positive cathode right behind the sample, and the negative anode in front of the sample. (Again, take a look at the diagram.) Of course, the positively charged polymers are going in the direction of the anode, attracted to its negative charge. If we play it right, we can use this acceleration to shoot the polymer molecules down to the detector at the far end of the chamber.
Most of the time, there is only one single positive charge on each polymer molecule. This means that the same electrical force is applied on each polymer molecule when it's being accelerated in the electric field between the two electrodes. But remember, the polymer molecules have different masses.
What did Isaac Newton say about mass, force, and acceleration? I think it went something like this:
F = ma
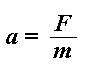
So the polymers will hit the detector, the small ones first, then the big ones. They hit completely in order by mass. All the polymer molecules of the same molecular weight will hit the detector together. When they hit the detector, the detector registers a peak. The size of the peak is proportional to the number of molecules that hit at one time. So when we're done we get a series of peaks that looks like this:
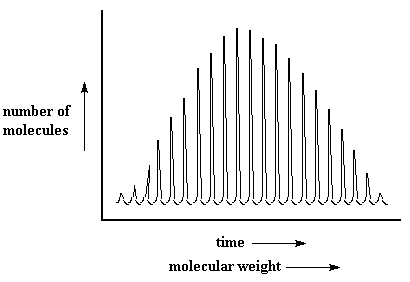
I don't know about you, but that MALDI spectrum has always reminded me of the giant fin on the back of a dimetredon, an extinct reptile that lived millions of years ago.
MALDI and SEC
One little detail you may notice is that molecular weight increases from left to right on this plot. On an SEC plot, which you see more often, molecular weight increases from right to left. (Can you figure out why?) This can make things confusing at times, so keep it in mind.But there are more important differences between SEC and MALDI. SEC gives you an approximate molecular weight distribution. SEC measures hydrodynamic volume, not molecular weight. We can then approximate molecular weight, by comparing the hydrodynamic volume of the polymer we've tested to a standard, usually polystyrene, for which we know the exact relationship between hydrodynamic volume and molecular weight. This only gives an approximate result because the relationship between molecular weight and hydrodynamic volume is not always the same for every polymer.
But MALDI measures the mass more accurately, because it doesn't compare the polymer you're measuring to anything. It gives an absolute measurement of mass. Given time MALDI will probably replace SEC in most laboratories.