Modern Contact Lenses
Materials made of "schizophrenic molecules"
Use your mouse on the model to rotate and zoom.
Click items below for other modifications.
spin on spin off
spacefill wire thick wire ball&stick
dots: Vanderwaals dots off
Modern polymeric contact lenses are constructs that breath and feel good on your eye. It wasn't always so and it's taken years and the efforts of hundreds of researchers to develop the ones sold today. Why so long? Turns out it's not a simple problem or one that's easy to solve. The reason is simple, however: we ask that a contact lens do multiple things at the same time, things that are normally "mutually exclusive." That is, we need properties that were never found to go together before, either in man-made materials or ones we find in nature.

The diversity of molecular structures and properties is enormous, perhaps even infinite. We have gradually learned how to use that diversity to solve everyday problems. Take soap, for example. Most soap molecules are a combination of two types of chemical structures that really don't like each other, some might call it hate. While 'hate' may be too strong a homocentric word, 'incompatible' certainly fits the bill.
To oversimplify some, one part of a soap molecule loves water, while the other part doesn't, and in fact, loves grease and dirt. That part binds or absorbs the dirt on your hands, clothes or dishes and hold onto it tightly. The other part, however, really wants to be surrounded by water molecules, so what happens? The 'dirt' part is hidden inside a shell of the dirt-loving parts while the water-loving parts suck the whole mess (called a micelle or emulsion) into water. Joilla! Something gets cleaner! To put the molecular behavior in more scientific terms, one part is hydrophilic and the other part hydrophobic.The technical term for the chemical display of "dual personalities" is amphipathic.
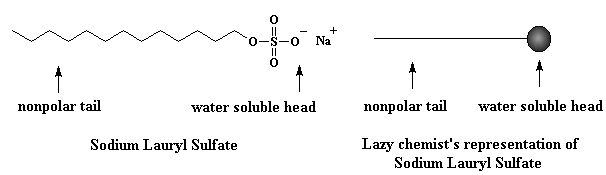
One soap molecule isn't much good to you. But when you get a whole bunch of 'em together, neat stuff starts to happen. At a certain concentration in water, soap molecules congregate and form micelles. Scientists have an apt (if not original) name for this called the critical micelle concentration, or CMC for short. Don't let the scientists fool you; these molecules are really doin' the hokey pokey, tails inward.
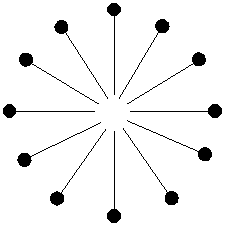
Any dirt, grease, or grime that you happen to have on your hands is most likely organic and looks like this (ok, so it doesn't really look like that but just use your imagination):

When you wash your hands with soapy water, the hokey pokey party really gets going. The insoluble dirt particle jumps right in the middle of the soapy micelle where it's pretty happy. It doesn't want to get out so it stays dissolved in the organic tails of the soap molecules.
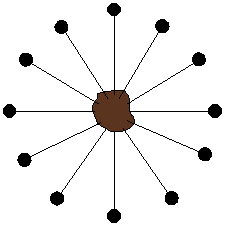
Some typical methacrylate monomers are shown above, including the methyl ester and the hydroxyethyl ester monomers. The latter (HEMA for short) found use in some of the more comfortable contacts made after people complained about having a piece of plastic stuck to their eyeballs. PolyHEMA is water soluble, although with some crosslinking monomers added (such as the EGDMA shown below), the lenses swell with water and become very soft. While this is great for comfort, it doesn't help much getting oxygen to the eyeball, something very important since eyes have so little blood flow that they can't get much oxygen in the normal way, from blood. In fact, eyeballs actually "breath" when there's nothing covering them up. But wait! Isn't that what we're doing when we put a contact lens in our eye? Problem!
Over the years (decades, actually) since that first contact lens was made, scientists have worked hard to make lenses that could transmit oxygen from the air into the eye. They also worked at making the lenses more comfortable. Turns out, those things are pretty much mutually exclusive because what helps the one, hurts the other. Why?
One way to increase comfort is to "just add water." The more water in these so-called "hydrogel" lenses, the more comfortable on the eye. Problem is that water is pretty lousy at transmitting oxygen to the surface of the eye. One problem solved but the other not.
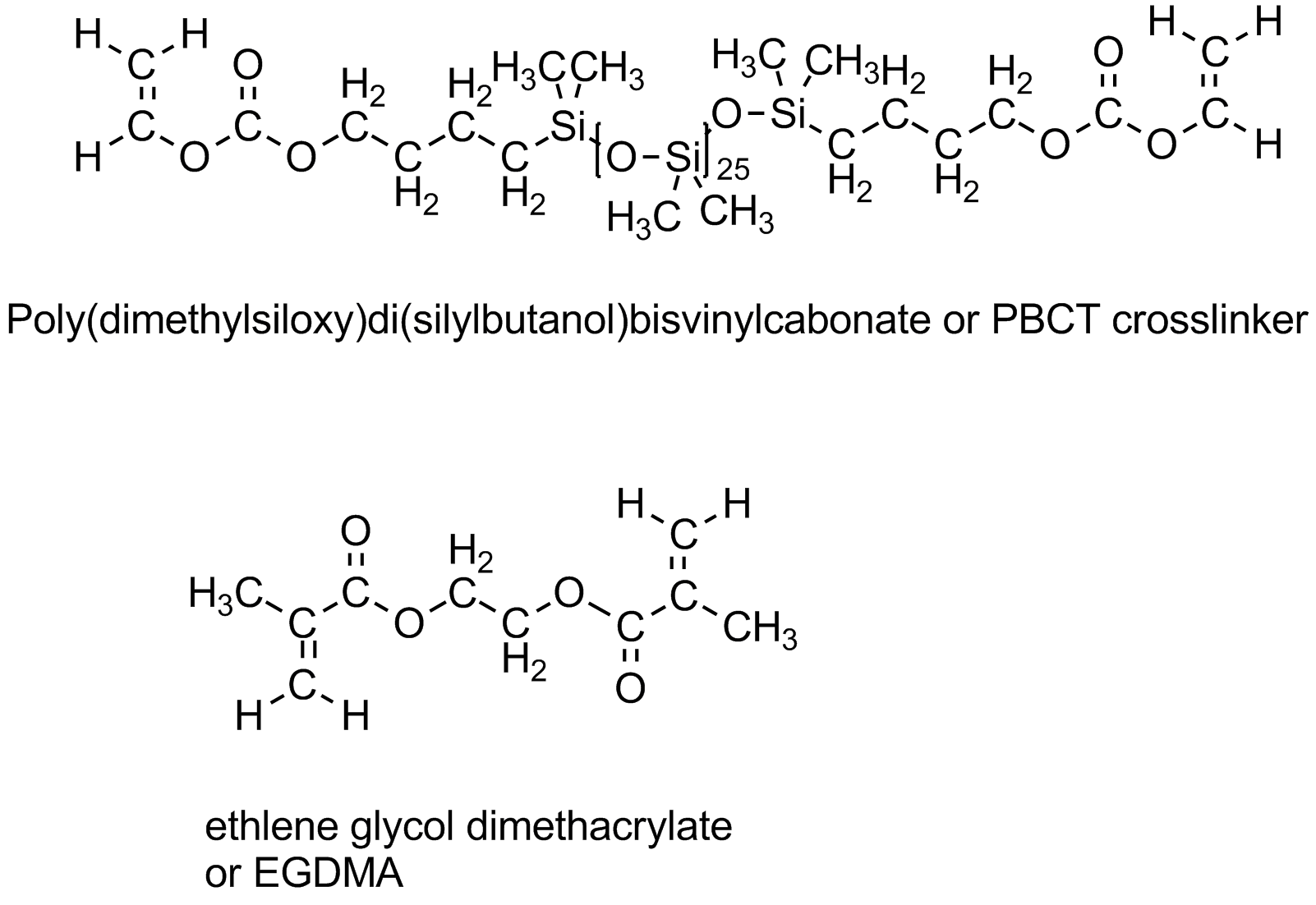
How to increase oxygen transport? Turns out there are a couple of ways of doing this, both pretty expensive since they involve uncommon polymer structures. Those structures contain either fluorocarbon segments (mini-me Teflon, as it were) or siloxane groups (just like silicone bathtub caulk that keeps water and mold from getting in all the cracks and crevices around your tub or shower). Yeah, you guessed it: both really don't like water. At all. As in, you can't even wet them with water, it just beads up and runs off. Some typical siloxane monomers are given below. They go by the shorthand name of "Tris" which relates to the tris(trimethylsiloxy)silane group. (Go to the top of this page to see the TRIS monomer in all its 3D glory.)
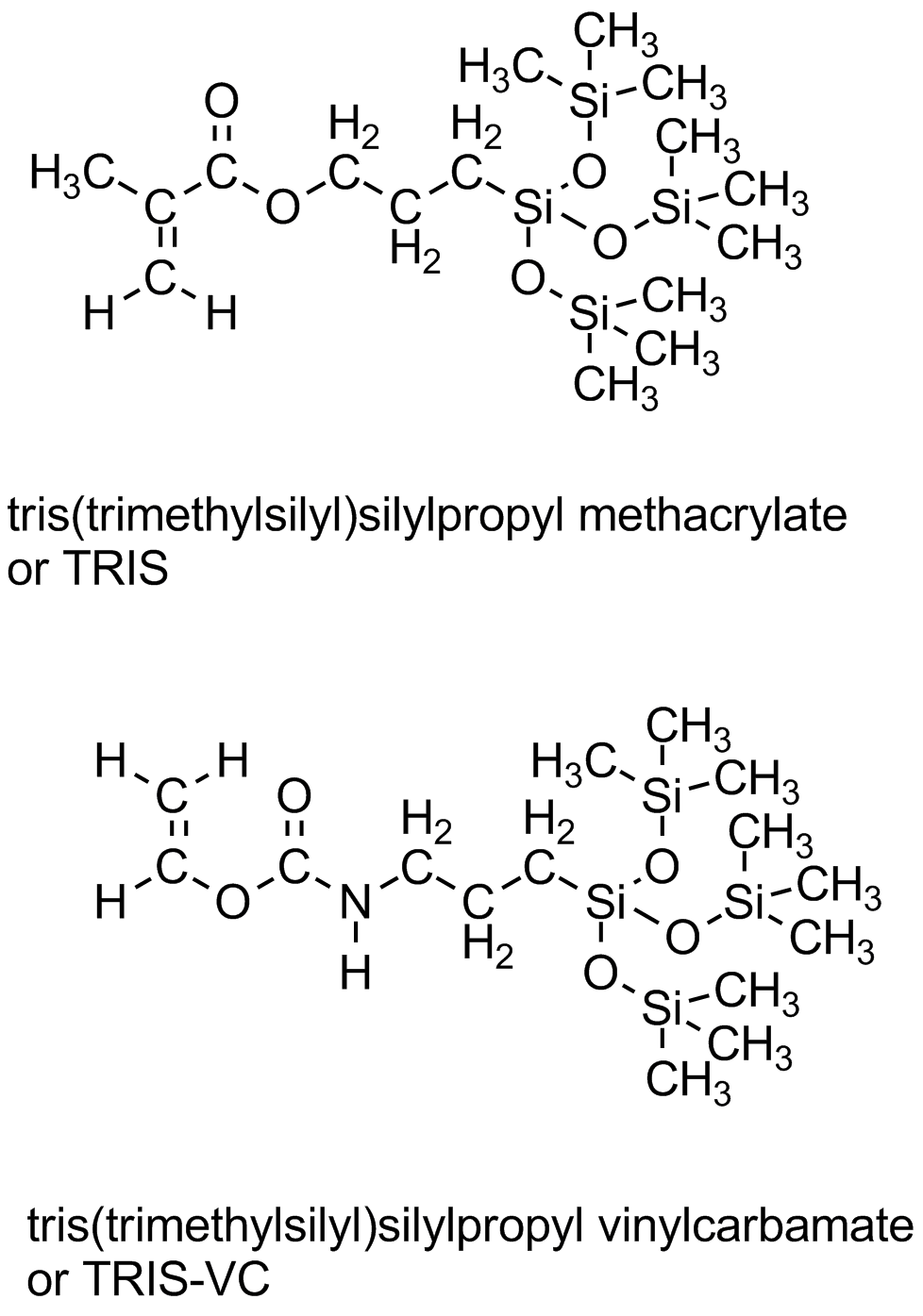
So if you make a contact lens from such polymers so that they have really good oxygen transport, the eye might be healthy but you won't wear the lenses. Why? Because they feel like a piece of something stuck to your eye. In fact, such hydrophobic lenses can get so tightly stuck on the surface of an eyeball that when you finally pull one off, you rip off some of the eye. Seriously not good. Other typical monomers used in earlier contacts are given below. Mostly pretty simple monomers and even in combinations of various kinds and amounts, they didn't really solve the problem of conflicting property requirements.
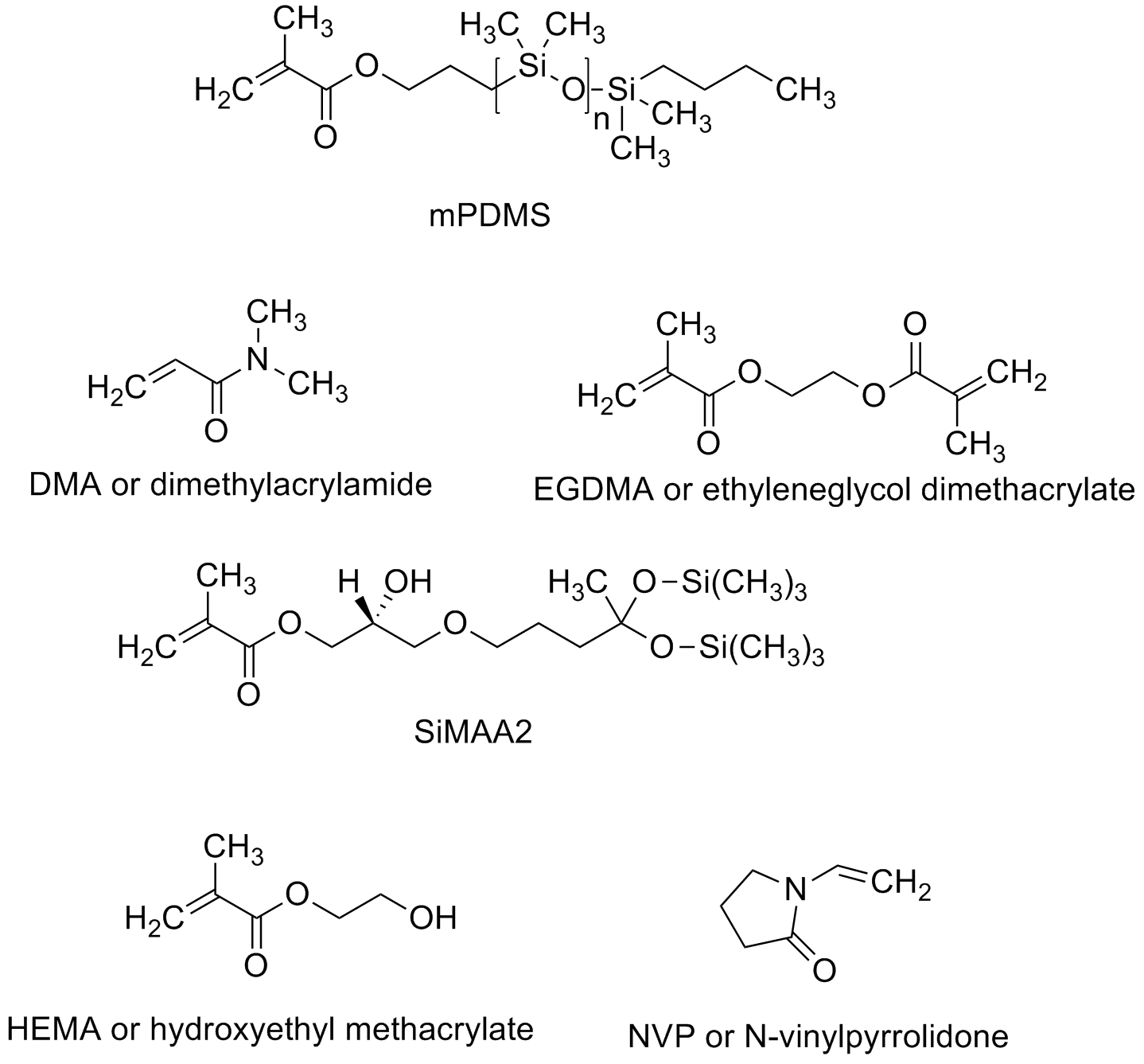
Here's the problem: water-loving polymers feel good but water-hating ones transport oxygen better. Hydrophillic polymers like polyoxyethylene and poly(N-vinylpyrrolidone) make very comfortable lenses BUT that you have to take them off fairly often to let your eye "breathe." Fluorocarbon and siloxane polymers make great breathable lenses that you have to take off fairly often also (if you can) because they feel so bad on your eye. What to do, what to do...
Aha! You say. Combine the two just like we (using the term in a strictly human race way) did with soap molecules. Obvious thing to do, but extremely difficult to do right. Again, why? These two types of polymers or segments or blocks still don't like each other even if you chemically link them together. They'll do anything possible to get as far away from each other as possible. And that's a kind of behavior you can actually use to make better lenses.
So it turns out that if you make a copolymer with both hydrophilic and hydrophobic segments (repeat units or blocks), those segments will spontaneously "phase separate," especially when the polymer is being made with a little water present. And besides, the lenses always get wet on your eye anyway since your eye actually "cries" all the time to remove waste material from the surface. Once the two types phase separate, they can't work together to be both comfortable and oxygen permeable. The oxygen permeable segments are too small and discontinuous to provide a pathway all the way through the lenses for oxygen molecule transport. Worse, these parts are hydrophobic and have a surface energy that tends to bring them to the surface of the lens where they make it feel uncomfortable again despite all the water that might be absorbed inside the lens itself. Bummer!
But wait! There's more! What if we just crosslink the polymers so the segments can't phase separate? Great idea. Doesn't work. You still have nano-phase formation that blocks the oxygen transport and/or prevents the surface from getting wet and being comfortable. But, and this is a really big but, if you tune things just right, using the right ratio of the two types of monomers and the right kind of crosslinking monomer and the right amount of initiator and just the right amount of water or water-like solvent, why a neat thing happens: IPNs!
"Wow!" you say, "That's utterly fantastic! What's an IPN?" Great question and one it took us a long time to figure out how to get the answer to. IPN stands for "interpenetrating polymer networks." Clear, right? Ok, then, here's what it means: yes, phase separation occurs but because of the way the polymerization is done (especially with just the right kind of crosslinker present), you form two continuous phases that stretch all the way from one surface of the lenses to the other one. That means there's a clear path for oxygen to follow so it can get from the air to the surface of the eye. It also means that there's another separate path for water to fill up, and this allows transport of water-soluble stuff away from the eye surface PLUS makes for a water-filled layer right next to the eyeball so the lenses feel great on the eye.
Long story short: these kind of new lenses are now made by just about everyone making lenses. Sure, there are small differences between brands, and yes, we haven't discussed all the details of how to make sure the surface on the eye has just the right composition for comfort, but this is a good start. If you want to learn more about this are, you can find reviews that discuss last year's materials. But if you want the most recent developments, the ones using what's actually in the lenses on the market today, you have to read a whole bunch of patents and then decipher them to figure out what they actually mean. Good luck with that!