Aliphatic Polyethers
The model above is an image of the POM model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
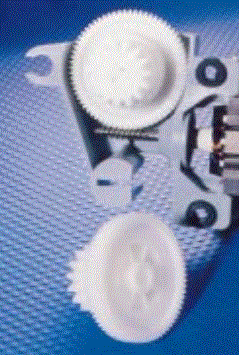
Polyethers are used as stand-alone polymers in a variety of important applications we'll discuss below, but take a look at the gears on the right made of POM. They're also used as the "soft segment" in polyurethanes. Plus when polyoxyethylene is grafted onto other polymers or surfaces, those surfaces become biocompatible, allowing use in medical devices.
Not to get the cart before the horse (which makes the cart very hard to steer), let's back up and talk about what we're going to talk about here. While there are lots of aliphatic polymers that contain ether linkages connecting the repeat units (cellulose and chitin for two of the more complicated ones), we're going to focus here on linear polymers that have just simple structures.
"So what do you mean by simple? Polymers seem to be pretty complex materials."
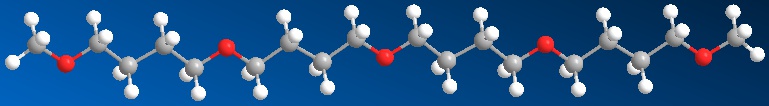
Right you are, but by simple, we mean just the bare necessities, as the song goes, to make a polymer backbone. Let's start with polyethylene. You can't get simpler than that, with a repeat unit containing just two carbons, each of which has just two hydrogens attached: -(CH2-CH2)-. Now replace one of the CH2 moieties with a simple oxygen, and you have the simplest aliphatic polyether, polyoxymethylene:
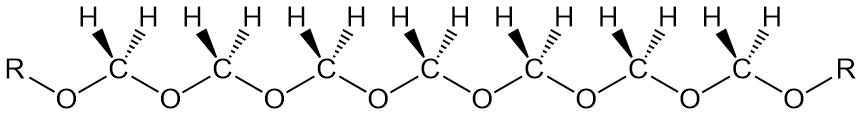
We'll describe this "wonder polymer" here a little bit, but if you want a more complete version, go here. The other two polyethers we'll talk about here are POE (polyoxyethylene) and poly(butylene oxide) (also called polytetrahydrofuran for reasons we'll discuss). The most common short names for these two polymers are PEO (as well as POE) and polyTHF or PTHF.
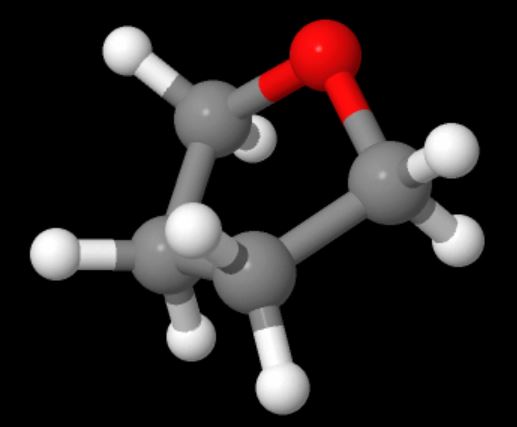
Now here's an interesting fact about these three polymers: they're all made (or can be made) from cyclic monomers. What's even weirder is that the sizes of two of the cyclics are either 5- or 6-memberd rings (see below; click on the colored images to pop up 3D versions). These are supposedly the most stable rings sizes you can have for simple hydrocarbons, and you'd guess that stability would apply even with one or more oxygens substituting for carbon. So what makes them unstable enough to ring open to give linear polymers?
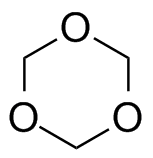
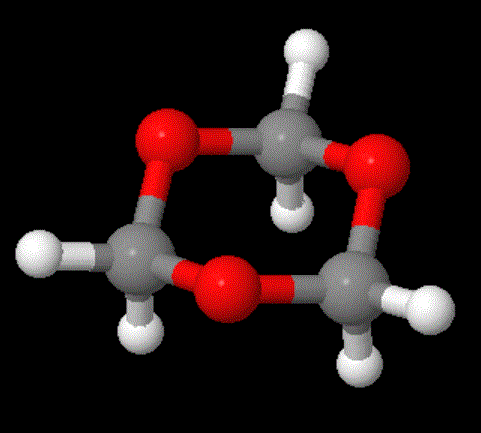
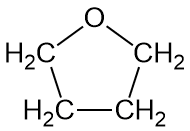
Don't forget that any chemical transformation must have some kind of driving force, some reason for it to take place. Usually that driving force is enthalpic, meaning there's a stability reason for it to occur: the product is more thermodynamically stable than the starting material. If the starting material is really stable, though (like 5- and 6-membered rings with only sp3 hybridized atoms), than there's no real reason for polymerization to take place. What gives here?
It always helps to compare things that are with things that aren't, things that do with those that don't (or shouldn't). So let's look at the monomer for POE (polyoxyethylene): it's a highly strained three-membered ring epoxide (shown below). We won't talk about how you make this very strained and very reactive monomer, but just about what it looks like.

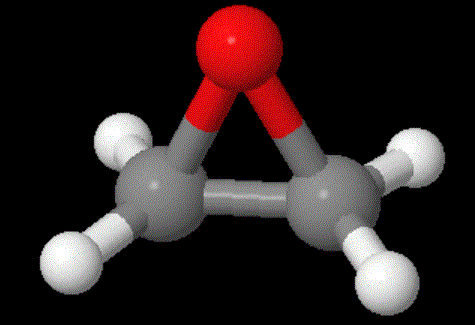
As you can see, the bonds between any three atoms in this ring are much tighter than those in the 5- and 6-membered rings above. The more tight (or strained) the bonds, the more unstable and reactive the compound is. Based on this simplistic discussion, the epoxide monomer should be really reactive. Guess what? It is! In fact, it's so reactive that you have to be careful to keep it cooled when making the polymer or explosive things can happen. But the good news is, we know how to make the polymer and it's shown below. Click on the structure to pop up the 3D version you can move around and zoom in and out.

Now for the really interesting name: polytetrahydrofuran. "Whoa!" you might exclaim, "That indicates the monomer is a cyclic five-membered ring with one oxygen atom and four carbons." Right you are! So take a look at the two images below; click on the bottom one to pop up a 3D version you can examine more.

Stability and Reactivity
"What's that you say?" you ask in total perplexity. "I thought you said that 5- and 6-membered rings were stable. Doesn't that mean they won't ring-open to polymerize?"
Yeah, well, stability is a relative thing, isn't it? Put a book on the table and it's stable, but not really. It really wants to fall to the floor. Slide it to the edge of the table, and "Whap!" down it goes. And here's the thing we haven't talked about yet: entropy! Any reaction has two parts to consider: enthalpy of reaction and entropy of the overall conversion. Enthalpy has to do with bond strengths and stabilities, and it seems that for cyclotrioxane and tetrahydrofuran, ring stability is pretty good. That means enthalpy isn't much of a driving force if it helps at al. But (and here that's a big but) entropy for ring-opening must be favorable, (the TΔS term below) and that helps drive the conversion of such cyclic structures to linear polymer.
And we don't have time to go into detail on why this is. Suffice it to say that there are different kinds of entropy: vibrational, rotational, translational and more. So in this case, it's most likely the increased freedom of segmental motion on forming the polymer chains. That is, parts of the ring (now in the polymer) can rotate and squirm around with greater ease. Kind of like a snake with its tail clamped in its mouth: not much wiggle room there. But when it opens its mouth and the tail pops out, why, now it can wiggle around and slither forward and do all kinds of snakey things like catching bugs to eat. Anyway, we'll talk about entropy and enthalpy in more depth later. For now the good news is, we CAN make polytrioxane (POM) and polytetrahydrofuran (PTHF) pretty easily and to high molecular weight. Great!
Now I'm sure everyone out there is just dying to have two questions
answered. The first one is:
So what? Why should I care?
And the second one which I'm positive everyone is wondering about is:
How are these polyethers used? Exactly what are they used for?
These two riveting questions, as it turns out, have the same answer. First, these three polymers all have glass transition temperatures that are really low. That makes two of them soft and pliable, POE and PTHF. But it turns out that even with a very low Tg, POM has such tight packing and organization of the chains that it keeps its rigidity right up to its softening point (somewhere around 170 oC). That plus it's inherent "slipperyness" is why POM is used in high wear applications like plastic bearings in photocopy machines and runner slides in drawers.
"What about the other two, POE and PTHF? What are they used for?" Great question. POE has the interesting property that it's soluble in both organic solvents and in water. It can also easily be functionalized at either or both chain ends. Low molecular versions find use in various soaps and detergents, where they help dissolve or suspend dirt and oil residue. And higher molecular weight polyoxyethylenes show up in creams and other beauty products. It's especially useful in water-soluble lubricants for skin-on-skin interaction (use your imagination here). Because POE is also bio-neutral (some say it's biocompatible), it finds use in medicine and biomaterials. For example, it's used in eye drops to lubricate dry eyes, and it's also found in many dissolving pills and medicines where it helps carry the drug to the right spot to be digested.

Really high molecular weight POE (also called polyox by some manufacturers) has the interesting property that it aligns in water solutions when pulled or pushed on. There are reports that you can make a self-syphoning solution of POE with molecular weights of over a million: you snag some of the thick solution, pull it up and over the side of the beaker and down below the beaker bottom, and it will continue to pull more out till the beaker is empty. Weird.
But the useful property related to this is in fire fighting. It's hard to push water high enough to attack fires in high-rise buildings. Put some of this very high molecular weight POE in the pumper tank, and you can shoot the water a couple stories higher. Wow!
"Ok, what about the other polyether you mentioned made from tetrahydrofuran?" Great question! Polytetrahydrofuran plays an important role in many condensation polymers like polyesters and polyurethanes. Because it's very flexible and has such a low Tg, it softens almost any polymer it's incorporated into. Polyurethanes especially benefit from PTHF segments between the urethane linkages in the backbone. The alternation of soft PTHF segments with harder urethanes makes such polymers "thermoplastic elastomers." That means they can be melt (or solution) processed but then form structures that are strong and tough. Clear wood and floor finishes are made of polyurethanes with PTHF segments, for example. It's also used in such important materials as "spandex," where the reversible stretchyness makes work-out clothes more comfortable. More important are the biomaterial applications where such polymers seal electronic devices put into the body and protect them from corrosion.
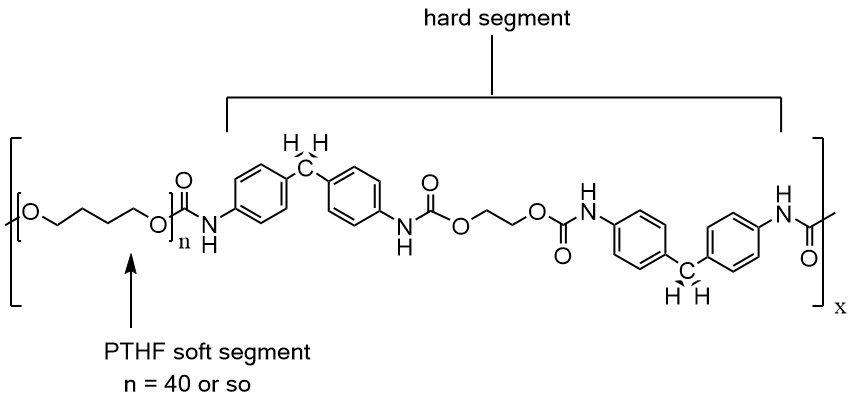
All-in-all, aliphatic polyethers have enormous impact on our daily lives. They impart toughness and strength to coatings and fabrics on one hand and on the other, make some of the most important biomedical devices available today functional without harm to human bodies. Someday you may find these polymers making your life better. When that day arrives, thank a polymer scientist!
Other polymers used as plastics:| |
Other polymers used as fibers: | |
| Polypropylene | Polypropylene | |
| Polyethylene | Polyethylene | |
| Polystyrene | Nylon | |
| Polycarbonate | Kevlar and Nomex | |
| PVC | Polyacrylonitrile | |
| Nylon | Cellulose | |
| Poly(methyl methacrylate) | Polyurethanes |

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |