
The model above is an image of the pdb model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
Poly(phenylene oxide), or PPO, is one of those high-performance polymers we like to call engineering thermoplastics. Its biggest strength is its resistance to high temperatures. It has a very high glass transition temperature, 210 oC. But there's a price for being heat-resistant. Most polymers are processed at high temperature in a liquid-like state. But if your polymer won't become liquid-like at reasonable temperatures, you can't process it! For this reason, PPO is often made into blends with high-impact polystyrene (HIPS for short). Blending PPO with HIPS makes the PPO easier to process, plus it gives PPO some resilience. PPO needs this toughening because by itself PPO can be brittle in some situations. General Electric makes PPO/HIPS blends and sells them under the name NorylTM.
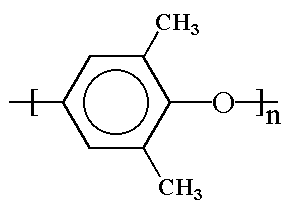
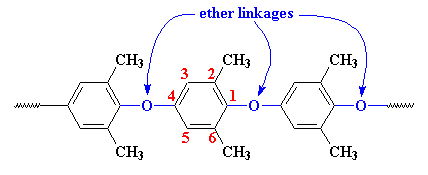
Structurally, PPO is made of phenylene rings linked together by ether linkages in the 1,4 or para- positions, with methyl groups attached to carbon atoms in the 2 and 6 positions.
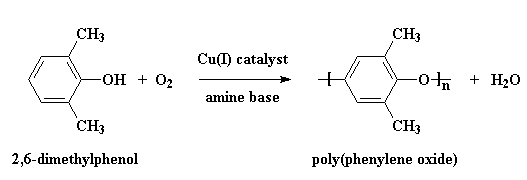
PPO is made by what we call oxidative coupling polymerization of the monomer 2,6-dimethylphenol. Water is a by-product, and so this is technically a condensation polymerization. It's also a step-growth polymerization, which tells you something about how fast it builds molecular weight and the general mechanism of reaction.
This is what our monomer 2,6-dimethylphenol looks like in 3-D:
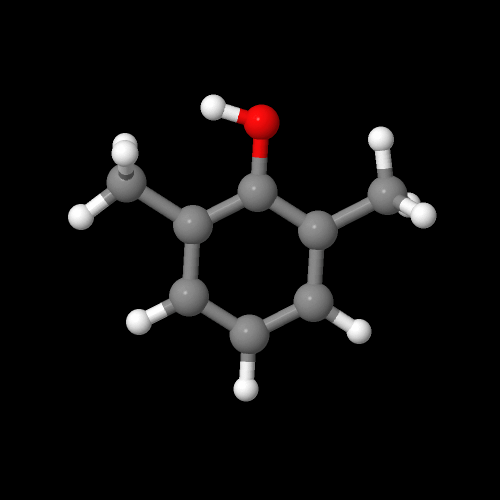
You can view the 3D image of DMP by clicking here or just click on the image itself.
Be sure to close the new window that opens up when you are ready to come back here.
Tested Syntheses of PPO Poly(phenylene oxide)
So if you'd actually like to make PPO the way it's made industrially and in the lab, we have two procedures for you to follow. One uses 2,6-dimethylphenol, very similar to what is used industrially. The other uses the 4-bromo derivative of this phenol. Both are safe and reproducible synthesis, as long as you're careful like always when working in the lab.Click here to see the procedure and here to download a copy.
NMR Spectra of PPO
You somehow find what you think is a sample of this wonderful polyether. Maybe you even made it yourself. How can you be sure that's what it is? You decide to get an NMR spectrum or two. But of course, you have to have an actual spectrum of this material to compare it to.So here's a 1H spectrum of PET and and here's its 13C spectrum.
We also have spectra of several other PPO analogs and derivatives. In fact, if you search the files here, you'll find some interesting spectra to compare to PPO spectra.
Some other engineering thermoplastics include:

Return to Level Two Directory

Return to Macrogalleria Directory
