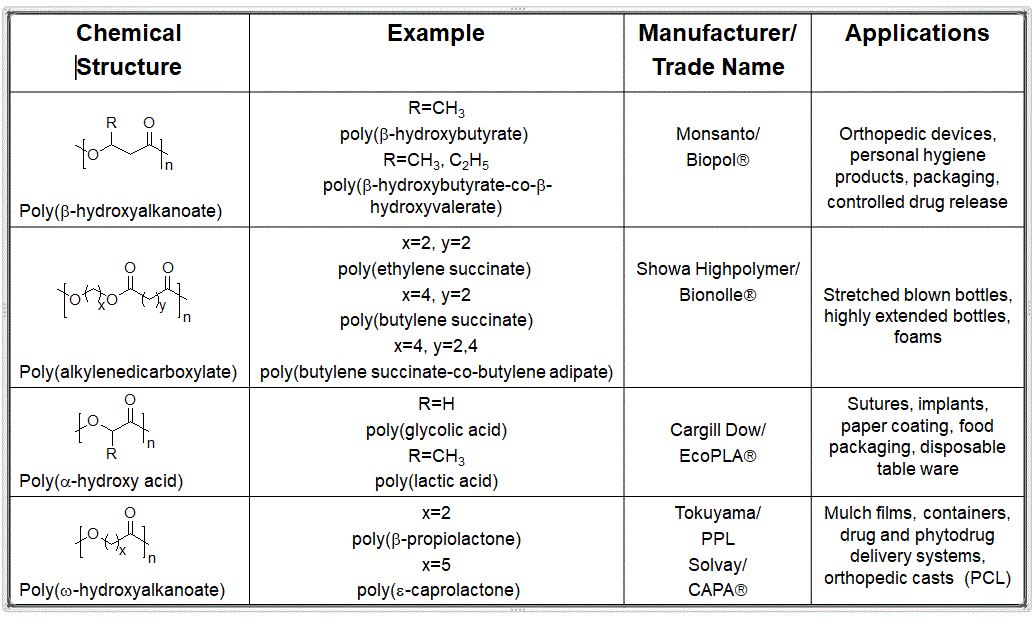
Turns out there are two main types of polyesters based on components in the repeat units that contribute to backbone flexibility or stiffness. Aliphatic polyesters are flexible, low Tg and Tm materials with reasonable physical properties. The table below lists some common aliphatics polyesters as of a few years ago. Note that some have changed hands or otherwise been relocated somewhere in the polymer universe.
We'll first explore the general properties of both families, focusing on the ester group hybridization and contribution to backbone behavior. Then we'll move onto aliphatic polyesters, discuss a few common examples, delve into the mechanisms of polymer formation and including monomer syntheses details.
The aromatic polyesters are by far the more important family commercially, used as fibers, films and optical materials in a variety of applications. We'll compare various members of this group by examining both properties and specific examples of commercial products. Then we'll describe polymer synthetic methods, both academic and industrial, with details of monomer sytheses and mechanistic considerations.
Last we'll discuss recent developments in polyester syntheses and new monomer contributions to properties. For example, we'll examine synthesis of PBT macrocycles and their use in ring-opening polymerization with improved control of kinetics, polymer molecular weight and properties. Then we'll describe all-aromatic polyesters (also known as "arylates") which require more demanding synthetic methods but give enhanced physical properties.
What's What and Where!
Back to what we mean when we use the word “polymer.” Most of the time when we talk of polymers we’re talking about plastics. And of course, you see plastics everywhere you look. As the old saying goes, "You can't swing a dead rat by the tail without hitting a plastic something or other," although why anyone would want to keep a dead rat around for such investigative purposes is beyond me...
Now for the reason you came to this section! To find out something about where polymers are actually used in everyday life. Well, we're going to kill two birds with one stone (another mysterious saying that we really don't want to pursue too deeply). Below you'll find the recycle symbols for six of the most common polymers in everyday use, plus the polymer that each represents. Also given are descriptions of several uses for each polymer.
To learn more than you ever wanted to know about the polymers in question, beyond what's on this list, just click on the name itself. Be advised, though, doing so will take you to our sister site, "The Macrogalleria," an exposition on many things polymer. And if you want even more examples of where polymers are used, visit Level 1 of the Macrogalleria where there are dozens of stores with polymer-containing things and stuff of all kinds. Just remember to follow your trail back here to continue with the last activity for this section: evaluation quizzes!
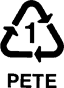 |
Poly(ethylene terephthalate): Soda bottles, water bottles, vinegar bottles, medicine containers, backing for photography film. And why PETE and not the more logical PET? Turns out a company making condensed milk already had a trademark of the abbreviation "PET" so it couldn't be used for recycle numbers. OOPS! Why PETE and not PET? |
 |
High-density Polyethylene: Containers for: laundry/dish detergent, fabric softeners, bleach, milk, shampoo, conditioner, motor oil, newer bullet proof vests, and all kinds of cheap toys. |
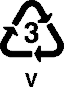 |
Poly(vinyl cloride): Plumbing pipes, shower curtains, meat wrap, cooking oil bottles, baby bottle nipples, shrink wrap, clear medical tubing (with tons of plasticizer added), vinyl dashboards and seat covers, coffee containers, plus knobs and switches of all kinds. |
 |
Low-density Polyethylene: Wrapping film for dry cleaning, grocery bags, sandwich bags, garbage cans, and again, cheap toys. |
 |
Polypropylene: Tupperware®, syrup bottles, yogurt tubs, diaper liners, outdoor carpet and rope. |
 |
Polystyrene: Coffee cups (foamed), disposable cutlery and cups (clear and colored), meat trays, "cheap" hubcaps, packing peanuts, and most important, styrofoam insulation. |
 |
The hotdog of plastics! Products labeled as "other" are made of any combination of 1-6 or some other, less commonly used plastic. So actually, the "7" doesn't mean much of anything useful. |
Is that all there is? Recycling?
Let's get one thing straight right now. That's definitely NOT all there is. Polymers have become so useful and so inexpensive compared to other types of materials that you'll find them in places you can't even imagine. Places like in your mouth and GI tract; in biomaterials used to rebuild or replace internal organs; in every vehicle used in personal and public transportation; and especially in industry and the military. It all comes down to "value," which can be defined as performance divided by cost. Cost seems to be the modern driver, not performance, so don't be surprised if some company sells you something that's really cheap but doesn't perform all that well. In general,then, there are too many uses of polymers in our world to list them all here. But continue on with the next few courses here and you'll find out more than you ever thought possible about polymers.
For now though, it's time for another (you guessed it!) quiz! Click here for your next evaluation quiz. It's a long one, covering more than what we've discussed in this course so far. But hey, that's a good way to learn other than by just reading a bunch of words on a page.
Good news! You've finished the introductory course on polymers- what they are, what they're made of, how they behave, and most important, where they're used. All good information that prepares you to dive into the details more deeply. The next course on Basic Polymer Synthesis will focus on the organic chemistry of making polymers. Key difference is this: a typical organic chemist is perfectly happy with a yield of 80 or 90%, but a polymer chemist has to have yields of over 99+%. "What?" you ask in amazement. "That can't be so, why, why, no organic reaction is that clean and complete."
Well, not to argue but in fact, it took us a long time to work how conditions and monomer purities that allow polymerizations to go to virtually 100% completion. Got your interest yet? Cruise on over to the synthesis course and find out more...