
Inside Look at Ionomers
Keywords
ion
An ionomer, as one might guess from the name, is an ion containing polymer. An ion, you might recall, is an atom or molecule that has an electric charge, either positive [+] or negative [-].) But an ionomer is more than just a polymer with ionic groups. We call any old polymer with ionic groups a polyelectrolyte. But ionomers are a special kind of polyelectrolyte. First of all, they are copolymers. They contain mostly nonionic repeat units, and a small amount of ion containing repeat units. How small? The ionic groups usually make up less than 15% of the polymer.
Wonderful. But you're talking abstractly.
Show me a real life ionomer, why don't you?
One example of an ionomer is poly(ethylene-co-methacrylic acid). This polymer is a sodium or zinc salt (which provides the counter ions) of copolymers derived from ethylene and methacrylic acid. Click on the second image below to see this copolymer in all its three-dimensional glory.
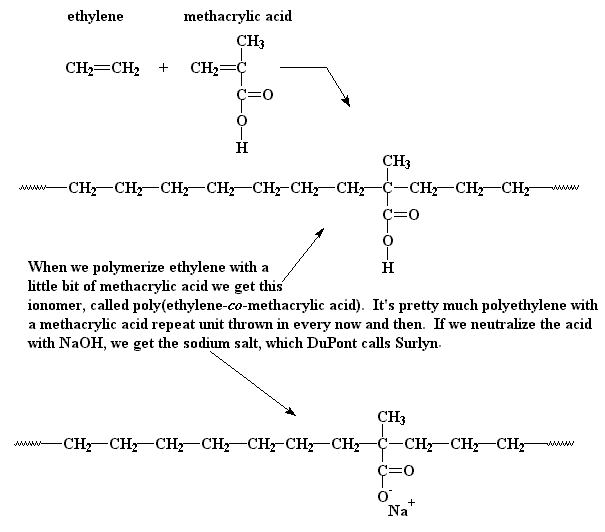
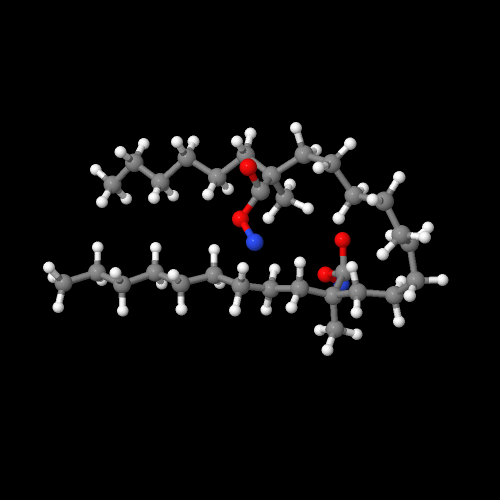
The model above is an image of the copolymer.
You can view the 3D image by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model
when you are ready to come back here.
The ionic attractions that result strongly influence the polymer properties. Look at the image above, or better yet, go to the 3D version and move that model around. Zoom in and rotate it so you can see how the two ionic groups ended up near each other after the energy minimization that was done on this short segment of the actual polymer. Also note that the polyethylene segments adopt a linear structure and come near each other, just like in pure PE crystallites. Models certainly help see things better, don't they?
The ionomer above is used as the cover of golf balls. Imagine being hit over and over again with a hammer shaped just like a golf club, and not tearing or breaking (unless, of course, the duffer makes a bad slice and cuts the cover open). Let's take a look at how ionomers work to have such fantastic properties.
In an ionomer, the nonpolar chains are grouped together and the polar ionic groups are attracted to each other. The ionic groups would like to go off into a little corner by themselves, but since they are attached to the polymer chain, they cannot do that in big clumps. Instead, they form very small clusters of just a few ionic groups surrounded by a sea of non-ionic polyethylene segments. This clustering acts like a physical crosslink and allows thermoplastic ionomers to act in ways similar to that of polymers crosslinked with chemical bonds or sometimes like block copolymers. Take a look.
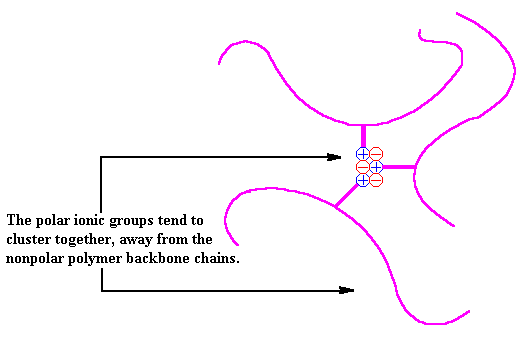
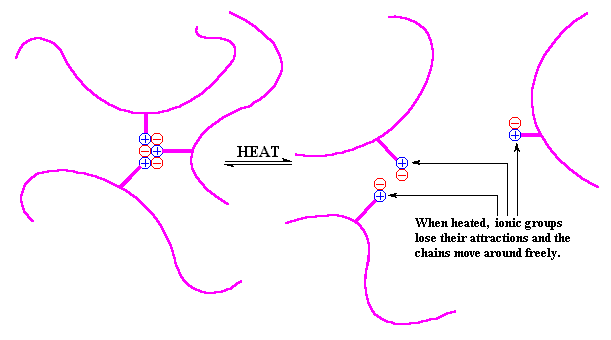
So far, we've only talked about what are called random ionomers. A random ionomer is one where the ionic groups are attached to the backbone chain at random intervals. However, there are a few other types of ionomers. We don't have time or space to describe all of these other types, but hopefully we'll get to that in short order (and don't hold your breath for a couple of scrambled eggs with toast).
A major use of ionomers is in semi-permeable membranes. A semi-permeable membrane is a very thin piece of polymeric material that lets some materials pass through while keeping others from doing so. Membranes made with ionomers are called "ion-selective membranes" since they can separate ions. These ion-selective membranes work by letting water pass through and not the metal ions like you find in salt water.
That's great,
but what's it got to do with me?
One commercial ion-selective membrane is a perfluorosulfonate ionomer, which DuPont invented and calls Nafion. All perfluorosulfonate ionomers have outstanding chemical and thermal stability, plus they have the ability to absorb incredible amounts of water. Nafion membranes can be made into films or tubes and can be used in many caustic and dangerous processes. Some such processes are the production of chlorine by electrolysis of brine, regeneration of spent acids used in the chemical and drug manufacturing industries, and in fuel cells and electrodialysis cells for precipitating useful metals from sea water. So, had enough for now about these novel and useful materials? Pick another topic and learn more.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |