


Proteins are one of many types of natural polymers, and they are the most versatile by far. You name it, proteins do it.
What can proteins do? What can't they do!
So, what are proteins?
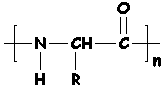
Proteins are polymers made out of amino acids. They're naturally occurring, meaning they're made by animals, plants, bugs, fungi, and other living things - and that includes you!
A protein is actually a polyamide (a what?), but more about that later.
So, proteins are polymers of amino acids. What's an amino acid? (Glad you asked!!)
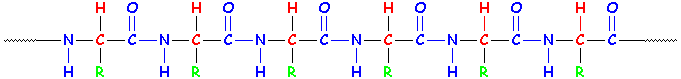
Amino acids have an amino end and an acid end. In the middle is a C (carbon) with an H (hydrogen) and a side group shown here as an R. (Think of R as the Rest of the molecule.)
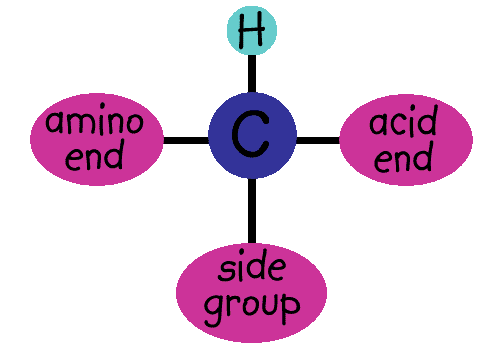
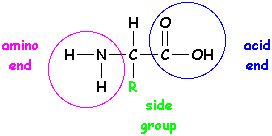
For each protein that's made, the order of the R groups is VERY important because it determines the shape of the protein and the job it will do. To get the right order, cells use RNA that's copied from your DNA. The whole story is pretty complicated, and involves special enzymes and even different kinds of RNA. We won't go into it now - just trust that there's a whole system in place to make sure the right amino acids get put together in the right order.
So, how does your body make a protein?
| 1 | |
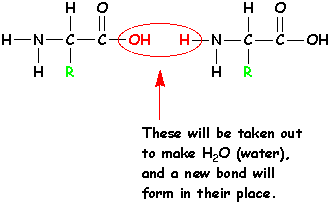
| First, hold 2 amino acids next to each other like this: | 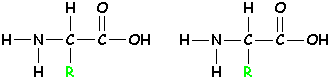 |
| 2 | |
|
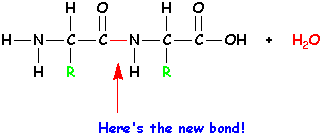
Take out the -OH and -H circled in red, and form a new bond. The -OH and the -H together make a molecule of water - H2O. |
|
 |
|
| 3 | |
| Bring in the next amino acid! | 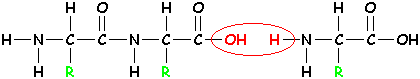
|
| 4 | |
| Again, remove H2O and form a new bond. | 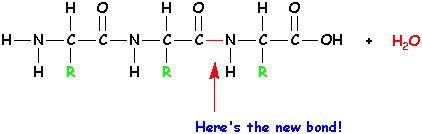
|
| 5 and up! | |
| Keep adding amino acids and taking out water until the protein is done! | 
|
Want to know something else? Proteins also make up silk. Silk is such nifty stuff that scientists tried to make synthetic silk. They tried to make synthetic polyamides, and what do you know, they did it! The artificial polyamides are called nylons.
O.k., earlier we said that a protein is a naturally occurring polyamide, and that we'd get to that later. Hey, guess what?! It's later!
A
Remember what our little baby protein looked like? Every time we added an amino acid, we made an amide group! See the amide groups in blue?
You want more natural polymers? Then check out these:
Here are two ways of looking at an amide group:
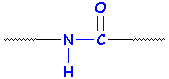
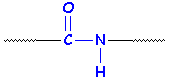

Now, we said that a protein is a polyamide. Any old polyamide would look like this, and the R groups don't have to look like the middle of an amino acid. It could just be a chain of six -CH2- units, or it could get more complicated.
Two Examples of Polyamides
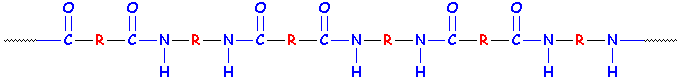
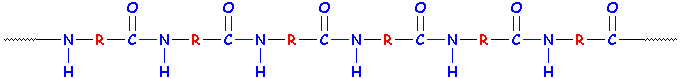
Notice something weird about those two examples? They're both general examples of polyamides, but their repeat units would be different (so they'd have to be made using different methods and different monomers, even if the R groups were the same.) Want to learn more about polyamides and nylons? Click here!

Return to Kinds of Polymers

Return to Main Page