CATIONIC POLYMERIZATION
-
Recomended Reading
- The polymerizations will be conducted at two temperatures, 0oC and -78oC. The 0oC is easily attained with an ice/water slurry. The -78oC bath is made up in a Dewar flask with dry ice used as the coolant. The liquid phase of the -78oC bath can vary depending on a number of considerations. Acetone/dry ice is perhaps the most widely used -78oC bath, however it suffers from bumping and the layer of dry ice at the bottom may make it difficult to put samples in for cooling. Propanol/ dry ice makes a viscous slurry which hold the temperature quite well and is not as prone to bumping as acetone.
- Six test tubes are carefully cleaned and thoroughly oven dried. While they are still warm they are capped with septum caps.
- The test tubes are numbered (this is easily done on the caps) and the following amounts of methylene chloride are injected into them: Tube 1, 30 ml; tubes 2-6, 40 ml. A second needle inserted in the septum cap serves as a pressure relief valve, when injecting liquids. The tubes should be weighed both before and after injecting the CH2Cl2 so the exact weight of CH2Cl2 is known.
- A hypodermic syringe is used to inject 0.9 ml (1.98 g or 7.6 x 10-3 moles) of SnCl4 into tube number 1. The weight should be checked before and after injection to obtain the correct catalyst weight.
- Purified styrene, 6.5 ml (5.9 g or 5.66 x 10-2 moles), is injected into each of tubes 2-6 and tube 6 is additionally injected with 0.02 ml of anisole.
- Tubes 1-3 are cooled in the 0oC bath and tubes 4-6 are cooled in the -78oC bath.
- When all the tubes are cooled (5-10 minutes), 4 ml of the SnCl4 solution is withdrawn from tube #1, injected into each of tubes #2 and #3, and the tube contents swirled. Weigh the syringe before and after injection to get the catalyst solution weight. When the injection in tubes #2 and #3 is completed, tube #1 is placed in the -78oC bath to cool. Start timing at the point of injection.
- After 10 min. has elapsed, tube #2 is removed from the ice, the septum cap removed and the contents poured into 100 ml of methanol is added to complete the precipitation. The product is filtered or decanted, washed with methanol, and left to dry until second week. The product is then weighed to the nearest 0.01g.
- When the initiator or catalyst solution has reached -78oC, (5-10 min.), 4 ml of the SnCl4 solution is withdrawn from tube #1, injected into tubes 4-6, and the tube contents swirled.
- Timing should be started at the point of injection.
- After 10 min. has elapsed, tube #4 is removed from the -78oC, bath, the septum cap removed and the contents poured into 100 ml of methanol with stirring. The contents are allowed to settled and a little more methanol added. If further precipitation occurs, more methanol is added to complete the precipitation. The product is filtered or decanted, washed with methanol, and left to dry until second week. The product is then weighed to the nearest 0.01 g.
- Tubes 3, 5, and 6 are removed after a one-hour polymerization time, and are precipitated and filtered as in steps 8 and 10. The same drying method should be used for all tubes in order that any errors caused by unremoved solvent will be relatively constant throughout the experiment.
- Prepare a 1g/100 ml solution of polymer in reagent grade toluene. Filter the solution in a fine porosity filter to remove any particulates.
- Use a Ubbelhode viscometer and a constant temperature bath (25oC) to measure the intrinsic viscosity. (Same procedure as the Dilute Solution Viscosity experiment.)
- Repeat the above procedure for at least three other concentrations.
- Determine % yield of the polymer.
- Calculate the molecule weight of each of the polymers produced using the Mark- Houwink Equation. (K and a for styrene in toluene at 25oC are 1.7x10-4 dl/g and 0.69 respectively) Show any plots necessary for your determination.
- Calculate
 n for the polymers produced.
n for the polymers produced. - In the reaction under study in this module the initiator system is SnCl4/H2O and the monomer is styrene. Show the mechanism of the co-catalyst and the initiation of the polymerization.
- Show the mechanism of the Friedel-Crafts alkylation reaction the may occur in this experiment.
- If the co-catalyst for the reaction in this module were ethyl chloride, what would be the reaction of the co-catalyst to provide an initiating species?
- You wish to polymerize 3-methyl-1-butene by cationic initiation. What would be the structure of the polymer repeat unit?
- Pearce, Eli M., Carl E. Wright, Binoy K. Bordoloi. Laboratory Experiments in Polymer Synthesis and Characterization. Educational Modules for Materials Science and Engineering Project, 1982.
- Odian, G. Principles of Polymerization 2nd Edition, Wiley-Interscience, New York, Ch. 5.
Objectives
Introduction
Experimental
Calculations
Post Test
References Pre-lab Quiz
Recommended Reading
Pearce, Eli M., Carl E. Wright, Binoy K. Bordoloi. Laboratory Experiments in Polymer Synthesis and Characterization. Educational Modules for Materials Science and Engineering Project, 1982 pp 83-106.
Cationic Vinyl Polymerization
The cationic polymerization of styrene, initiated by SnCl4/H2O is used to illustrate the effect of temperature on reaction rate and molecular weight and also the effect of Friedel-Crafts alkylation reactions on the molecular weight. Cationic polymerizations are initiated by electrophilic agents such as the halohydric acids (HCl, HBr, etc.), H2SO4, HClO4, etc. Lewis acids, which are electron acceptors by definition, and compounds capable of generating carbonium ions can also initiate polymerization. Examples of Lewis acids are AlCl3, SnCl4, BF3, TiCl4, AgClO4, and I2. Lewis acid initiators required a co-initiator such as H2O or an organic halogen compound. Initiation by Lewis acids either requires or proceeds faster in the presence of either a proton donor (protogen) such as water, alcohol, and organic acids or a cation donor (cationogen) such as t-butyl chloride or triphenylmethyl fluoride. Actually, the protogen or cationogen is referred to as the initiator while the Lewis acid is the co-initiator, since the protogen or cationogen ultimately supplies the proton or cation which adds to monomer that initiates polymerization. The propagation reaction in the styrene/BF3/H2O system is through repeated addition of monomer. Termination processes in these systems occur by transfer. For example, transfer of a proton to counterion, monomer, polymer, solvent, or some other added reagent can terminate the reaction. Transfer to counterion in the styrene/ BF3/H2O system is illustrated by This regenerates the original initiating species, which can then reinitiate polymerization as an active catalyst. An interesting phenomenon in the cationic system is the overall rate of polymerization and its relationship to temperature. Cationic polymerizations are normally conducted at low temperatures, and tend to go explosively fast in some cases. In most reactions, it is common for the rate of the reaction to increase with an increase in temperature. However, this is not the case in all cationic polymerizations. Considering a kinetic treatment of the polymerization of styrene/ BF3/H2O the overall rate of polymerization is given by
Thus, the rate is directly proportional to a combination of rate and equilibrium constants. The rate constant of a reaction is related to the activation energy for the process by the Arrhenius equation, where A is a constant called the pre-exponential factor, Ea, is the activation energy, R is the gas constant, and T is the absolute temperature. Using Ea(i), Ea(p), and Ea(t) to denote the energies of activation for initiation, propagation and termination respectively. For a overall rate constant for the polymerization, Since the Ea's and k's have a logarithmic relationship, the activation energy correspondingly would be If Ea(t) is greater than Ea(i) + Ea(p), the overall activation energy for polymerization would be negative and the overall rate would increase with decreasing temperature. This relationship of the energies of activation is encountered in many cases, since in initiation and propagation a dipolar species approaches a neutral molecule, a low energy process, while for termination an ion a pair is required to break apart and
rearrange, a high energy process. Furthermore, since the number average degree of polymerization, Xn, can be related to the rate constants as the Xn and consequently the molecular weight of the sample would increase with decreasing temperature if
Ea(t) > Ea(p).
Solvent effects on the counterion can also affect the reaction. Large increase in the rate and degree of polymerization are generally observed as the solvating power of the reaction medium increases. The effect of solvent is generally considered to manifest itself in two ways. The free ion concentration increases with increased solvating power, and Rp increases since the free ion propagates faster than the ion pair. Also, increasing the solvating power of the reaction medium should change the nature of the ion pair from the intimate ion pair to the solvent-separated ion pair. Objective
Introduction
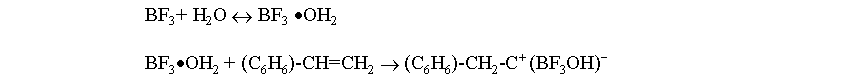
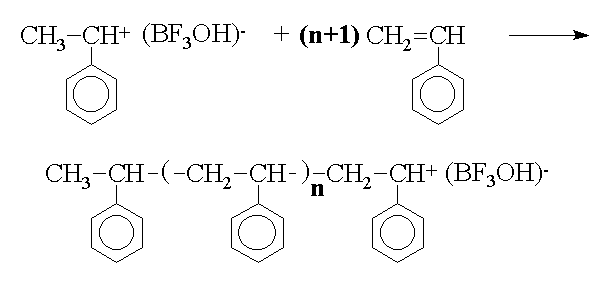

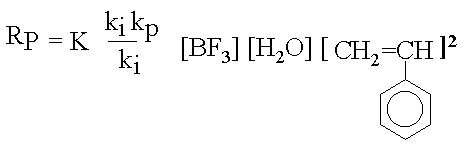


Experimental
First Week
Second week
Calculations
Post Test

References
Return to Anionic/Cationic Polymerization