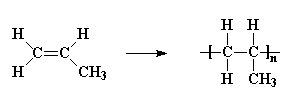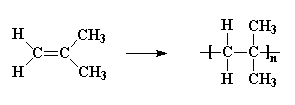Vinyl Polymers: Basics
Vinyl polymers are polymers made from vinyl monomers; that is, small molecules containing carbon-carbon double bonds. They make up largest family of polymers. Let's see how we get from a vinyl monomer to a vinyl polymer using for an example the simplest vinyl polymer, polyethylene. Polyethylene is made from the monomer ethylene, which is also called ethene. When polymerized, the ethylene molecules are joined along the axes of their double bonds, to form a long chain of many thousands of carbon atoms containing only single bonds between atoms.
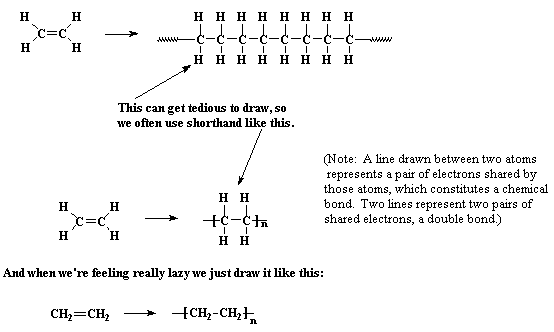
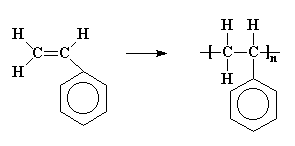
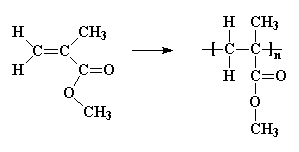
More sophisticated vinyl polymers are made from monomers in which one or more of the hydrogen atoms of ethylene has been replaced by another atom or groups of atoms. Let's see what we can do by replacing just one of those hydrogen atoms. We can get a number of common plastics:
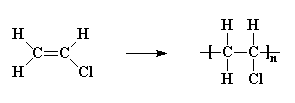
Replacing two hydrogen atoms, on the same carbon atom, we can
get
polyisobutylene, which is type of rubber.
Not many monomers in which hydrogen atoms have been replaced on both carbon atoms will polymerize. But one polymer that is made from a monomer substituted on both carbon atoms is polytetrafluoroethylene, which DuPont makes and calls Teflon.
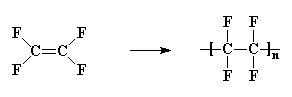
Vinyl polymers are made from vinyl monomers in a variety of ways, like:
- free radical vinyl polymerization
- anionic vinyl polymerization
- cationic vinyl polymerization
- Ziegler-Natta catalysis
- metallocene catalysis polymerization

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |