Poly(ethylene-co-methacrylic acid), a.k.a. Surlyn�.

In 1839 Charles Goodyear invented a process called vulcanization, which made rubber more durable and more useful at hot and cold temperatures. Vulcanization crosslinks rubber. That is, it ties all the chainlike rubber molecules together to form one big molecule.
This does a lot for the rubber, but it can also make rubber difficult to process. Think about it. A material made of one molecule can't really flow into a mold, and it can't be shaped or worked very easily. A material made of crosslinked rubber has to be shaped before it is crosslinked. Crosslinking also makes rubber difficult to recycle.
Some kinds of synthetic rubber aren't crosslinked, however. They can be molded and remolded again and again. They are called thermoplastic elastomers. Thermo- means "heat", and plastic means "moldable". Elastomer is just a fancy word that means "rubber." So a thermoplastic elastomer is a rubber that can be molded when it is heated.
While it is wonderful that thermoplastic elastomers don't have to be crosslinked, there is a question that hopefully some of you out there are asking. That question is:
Ionomers
One kind of thermoplastic elastomer is called an
ionomer.
An ionomer is a polymer that has a small number of ionic groups along its backbone chain. Not
many, just a few. For example,
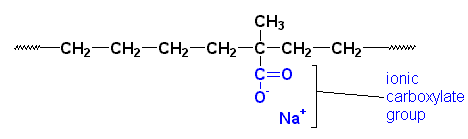
Surlyn� is a thermoplastic elastomer made by DuPont. It's used to make tennis shoe
soles and golf ball covers, among other things. Surlyn� is the sodium salt of a
copolymer
of ethylene and a small amount of methacrylic acid. It looks something like this:

Those ionic carboxylate groups do something for this material. The ionic groups tend to cluster together. They do this because they are very polar. most of the material is very nonpolar. Polar and nonpolar substances don't mix. Oil and water separate, but if one material has polar and non-polar groups in the same molecule, they can only separate so far. The carboxylate groups try to separate by clustering together. The result looks something like this:
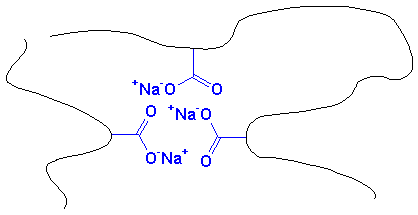
These clusters are held together very tightly. So tightly that the polymer chains act as if they were crosslinked.
That is, they act as if they're crosslinked until one heats the material. When the material gets hot the clusters break up. Then the material can be processed and molded.
Block Copolymers
A block copolymer is a polymer that has more than one section, or block. Here's an
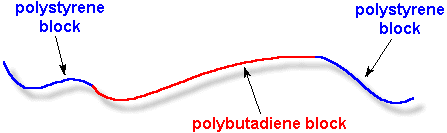
example, called
styrene-butadiene-styrene, or SBS rubber. A chain of SBS rubber is made of a short block of
polystyrene
followed by a longer block of
polybutadiene,
followed by another short block of polystyrene, like this:
Of course, polystyrene and polybutadiene homopolymers don't mix, just like oil and water don't mix. The short polystyrene blocks will clump together, trying to get away from the polybutadiene blocks. So we end up with chunks of polystyrene which are all tied to each other by the polybutadiene blocks, like this:
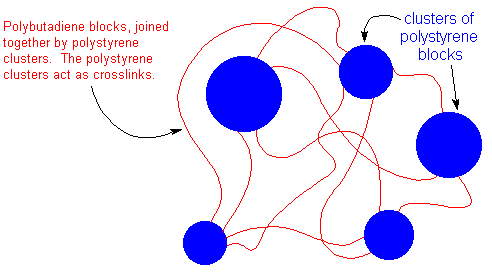
The polystyrene clusters act as crosslinks to tie all the polybutadiene blocks together. But unlike regular crosslinks, the polystyrene clusters come apart when the material is heated, so that SBS rubber can be molded, and recycled, too.