
Then there comes onto the scene a tinkerer named Charles Goodyear. In the winter of 1839 Goodyear was in Massachusetts trying to figure out how to make natural rubber more useful so that he could finally make a living at his until-now fruitless tinkering. He had mixed rubber latex and sulfur together when he had a little accident. He spilled the mixture on a hot stovetop. When the mixture was through frying, Goodyear couldn't believe what had happened.
Wouldn't you know sulfur was just the extra ingredient he needed to make rubber work in cold weather. After mixing hot gooey rubber latex and sulfur and letting the mixture cool, he took the rubbery solid that resulted and tacked it to the outside of his door. The cold Massachusetts winter didn't make it brittle. What's more, it didn't become gooey when heated anymore, either. Goodyear was onto something here. This process for making rubber more useable became known as vulcanization.

|
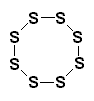
| A sulfur (S8) molecule. |
What Goodyear had done was this: he crosslinked the rubber. Let me explain. The sulfur molecules each contain eight sulfur atoms, arranged in a ring, like you see on your right. When these sulfur molecules are heated with polyisoprene molecules, something nifty happens. The sulfur rings open, and fall apart. Fragments of the sulfur rings will join with the polyisoprene, joining the chains together, as you see below:
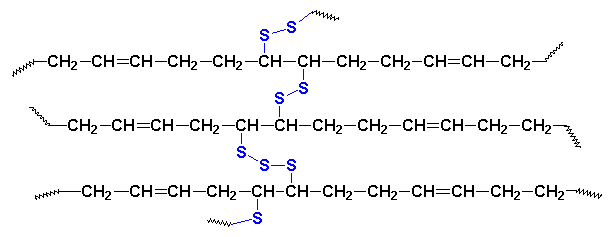
This crosslinking makes the rubber stronger. It also allows the rubber to keep its shape better when it is stretched over and over again. It keeps the rubber from getting gooey in hot climates because, think about it, a single molecule can't flow like a substance made up of many molecule. Think of the way you can pour a bucket full of gravel, but you can't really pour a boulder, and you'll get the idea.
The Drawbacks of Crosslinking
Now there are some drawbacks to this crosslinking which makes natural rubber so useful. First
of all, because it doesn't get gooey and flow when it gets hot, one has to mold it into whatever
shape one wants before crosslinking. But that isn't a really big problem, just something
for an engineer to keep in mind when making things out of natural rubber. But it's related to
a bigger problem. Because rubber doesn't flow when hot after it has been crosslinked, it is
very difficult to recycle. This is a big problem. Just think of how many tires are used up each
year by all the cars in the world. That's a lot of waste to dispose. Several experimental
processes are being investigated for recycling crosslinked rubber. Another answer is to use
certain kinds of rubber which aren't crosslinked called thermoplastic elastomers.
Next stop: Thermoplastic Elastomers
Polymer Science Learning Center and
the Chemical Heritage Foundation