By now you have probably guessed that a polyimide is a polymer that contains an imide group.
I just knew you were going to ask that. An imide is a group in a molecule that has a general structure which looks like this:
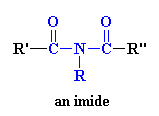
So if the molecular segment shown above were put into a polymer, the product would
be, you guessed it, a polyimide.
Polyimides usually take one of two
forms. The first of these structures is
a heterocyclic structure where the imide group is part of a cyclic unit in
the polymer chain. The second is a linear structure where the atoms of the
imide group are part of a linear chain. Take a look:
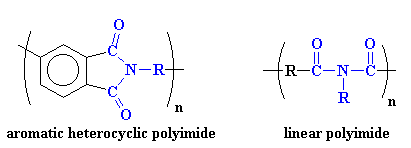
Aromatic heterocyclic polyimides, like the one on the left, are typical of most commercial polyimides, such as Ultem from Sabic (originally invented by G.E. Plastics) and DuPont's Kapton resin. These polymers have such incredible mechanical and thermal properties that they have replaced some metals and glass in high performance applications in the electronics, automotive, and even the aerospace industries. These properties come from strong intermolecular forces between the polymer chains. These forces involve polar interactions, aromatic stacking and most important here, charge transfer complexation.
A polymer which contains a charge transfer complex consists of two different types of segments, a donor and an acceptor. The donor is like a rich man with more money than he knows what to do with and wants to give some away. It has plenty of electrons to go around because of its nitrogen groups. The acceptor, then, is like a mooching houseguest. Its carbonyl groups, like our houseguest's many vices, sucks away its electron density. The donor doesn't mind supporting the acceptor, and in fact, with the acceptor around, the donor looks better. Charity looks good in some social circles (and it's good for a tax break too!). So the donor lends some of its electrons to the acceptor, holding the two segments tightly together.
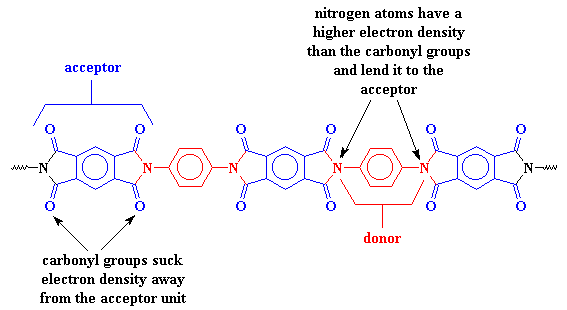
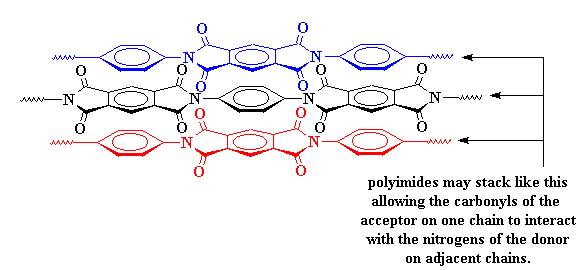
This charge transfer complex holds the chains so tightly that they can't move around very much. When things can't move around on the molecular level, they can't move around in the whole material. This is why polyimides are so strong. It's also why they don't melt or soften except at really high temperatures.
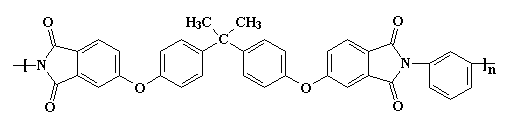
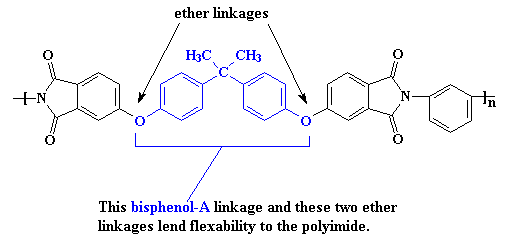
Of course, you can have too much of a good thing. The interactions are so strong, it makes the material difficult to process or do anything with. So it may become necessary to make the polymer a little softer so it can be made into something useful. This is accomplished at the molecular level by using monomers that have more flexible segments. One such is the bisphenol-A derived linkage shown in the polymer segment below.
Another interesting property of polyimides which makes them excellent for use in construction and transportation industries is how they burn, or rather don't.
Good question, and there's a good answer,too. It is not the burning which industry likes, but the way the burning stops. Polyimides are self extinguishing, which means they may start to burn but then quickly go out. On a molecular level this means that when flame touches the polyimide, a surface char develops which smothers it, blocking it from the oxygen needed for it to burn. Neat, huh?
Here are some other polymers used as thermosets:

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |