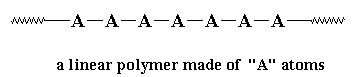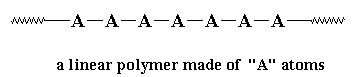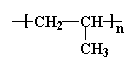This is sort of an introductory page for a more in-depth look at polymers. The first issue to get out of the way is this: when we speak of polymers, just what exactly are we talking about? What is a polymer and what isn't? Normally the word polymer is used when talking about molecules whose molecular weight (or size) is in the range of several thousand or more. More or less. Pretty simple, huh?
Get in Line!
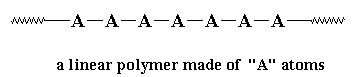
But usually we like to be more picky than that. Most of the time when we
talk of polymers we're talking about molecules with molecular weights
of hundreds of thousands, or even millions. We're also usually talking
about linear polymers.
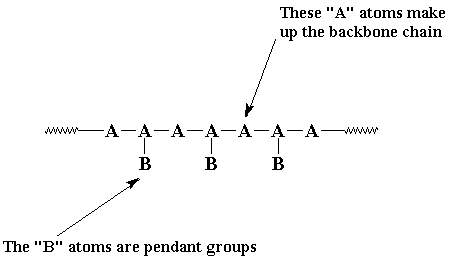
A linear polymer is a polymer molecule in which the atoms are
more or less arranged in a long chain. This chain is called the
backbone. Normally, some of these atoms in the chain
will have small chains of atoms attached to them. These small
chains are called pendant groups. The chains of pendant
groups are much smaller than the backbone chain. Pendant chains
normally have just a few atoms, but the backbone chain usually has
hundreds of thousands of atoms.
Polymers Are Like TV: Both Have Lots and Lots of Repeats
Normally, too, when we talk of polymers, we're not just talking
about huge molecules whose atoms are arranged in chains. We like to
think that the atoms that make up the backbone of a
polymer chain come in a regular order, and this order repeats
itself all along the length of the polymer chain. For example, in
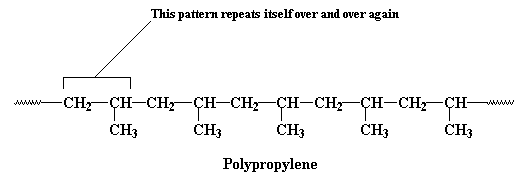
polypropylene,
the backbone chain is made up of just two carbon atoms repeated over and
over again. One carbon atom has two
hydrogen atoms attached to it, and the other has one hydrogen
atom and one pendant methyl group.
This unit of a carbon atom with two hydrogen
atoms followed by a carbon atom with a hydrogen atom and a methyl
group repeats itself over and over again along the backbone chain.
This little recurring structure is called the repeat structure
or the repeat unit.
To make things simple, we usually only draw one unit of the repeat
structure, like this:
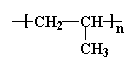
The repeat unit is put inside brackets, and the subscript n just
stands for the number of repeat units in the polymer chain.
Breaking the Line
Polymers can come in other structures, though. To find out,
take a look at the nonlinear polymer page.
The Consequences of Being Big
Let's get back to those simple linear polymers, now. These giant
chain-like molecules, because they are so big and because of their
shape, act in ways which small molecules don't. There are three
reasons for this. To find out what they are, take a look at the
page we call Three Things That Make Polymers
Different.
Some Assembly Required
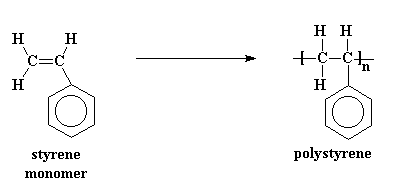
Polymers don't start out big. They start as little tiny molecules called
monomers. To make a polymer, a whole mess of monomers are strung
together in a line to form a long polymer chain. For example, styrene
monomers are joined together to make polystyrene:
For more on building polymers from monomers, go here.