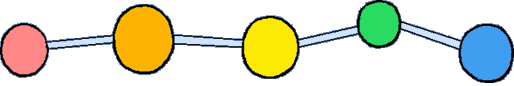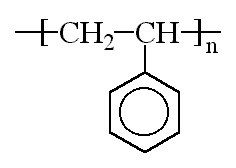
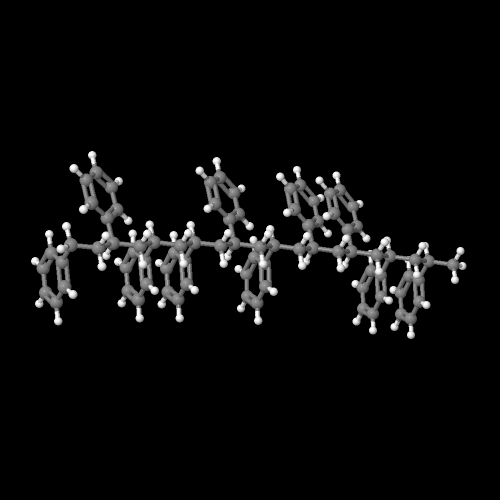
Polystyrene is a vinyl polymer. Structurally, it is a long hydrocarbon chain, with a phenyl group attached to every other carbon atom. Polystyrene is produced by free radical vinyl polymerization, from the monomer styrene.

This is a better picture of what the monomer styrene looks like:
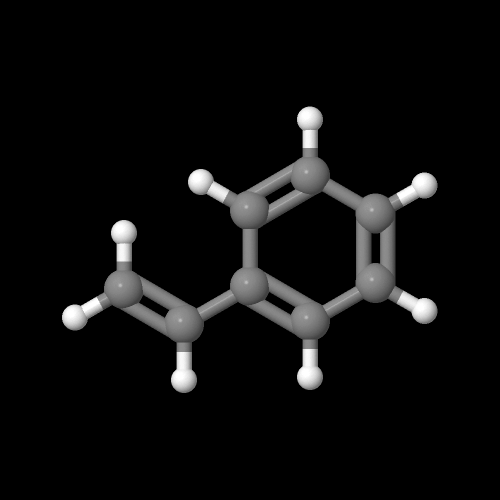
Go ahead, play with it!
Polystyrene is also a component of a type of hard rubber called poly(styrene-butadiene-styrene), or SBS rubber. SBS rubber is a thermoplastic elastomer.
There's a new kind of polystyrene out there, called syndiotactic polystyrene. It's different because
the phenyl groups on the polymer chain are attached to alternating
sides of the polymer backbone chain. "Normal" or atactic polystyrene has no order with regard to
the side of the chain on which the phenyl groups are attached.
The Polystyrene of the Future
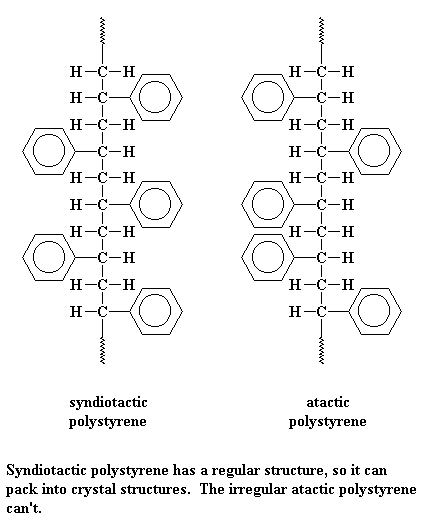
Syndiotactic polystyrene is made by metallocene catalysis polymerization.
But there are still some fun things you can do with old fashioned atactic
polystyrene. Wanna see something really nift-o-matic?
What would happen if we were to take some styrene monomer, and polymerize it free radically, but let's say we put some polybutadiene rubber in the mix. Take a look at polybutadiene, and you'll see that it has double bonds in it that can polymerize. We end up with the polybutadiene copolymerizing with the styrene monomer, to get a type of copolymer called a graft
copolymer. This is a polymer with polymer chains growing out of it, and which are a different kind of polymer than the backbone chain. In this case, it's a polystyrene chain with chains of polybutadiene growing out of it.
These rubbery chains hanging off of the backbone chain do some good things for polystyrene. Polybutadiene and polystyrene homopolymers don't mix, mind you. So the polybutadiene branches try as best they can to phase separate, and form little globs, like you see in
the picture below. But these little globs are always going to be tied to the polystyrene phase. So they have an effect on that polystyrene. They act to absorb energy when the
polymer gets hit with something. They give the polymer a resilience that normal polystyrene doesn't have. This makes it stronger, not as brittle, and capable of taking harder impacts without breaking than regular polystyrene. This material is called high-impact polystyrene, or HIPS for short.
HIPS can be blended with a polymer called poly(phenylene oxide), or PPO. This blend of HIPS and PPO is made by GE and sold as
NorylTM.
Other polymers used as plastics include:
Go Ahead, Hit It Hard!
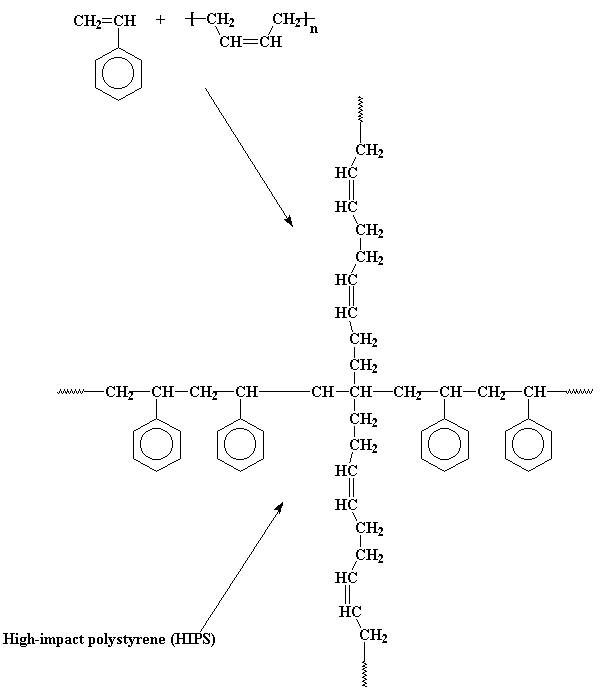
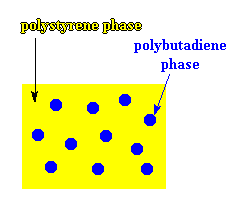 I'll let you in on a little secret. Not all the chains in HIPS are branched like this. There are a lot chains of plain polystyrene and plain polybutadiene mixed in there, too. This makes HIPS something we call an immiscible blend of polystyrene and polybutadiene. But it is the grafted polystyrene-polybutadiene molecules
that make the whole system work by binding the two phases (the polystyrene phase and the polybutadiene phase) together.
I'll let you in on a little secret. Not all the chains in HIPS are branched like this. There are a lot chains of plain polystyrene and plain polybutadiene mixed in there, too. This makes HIPS something we call an immiscible blend of polystyrene and polybutadiene. But it is the grafted polystyrene-polybutadiene molecules
that make the whole system work by binding the two phases (the polystyrene phase and the polybutadiene phase) together.

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |