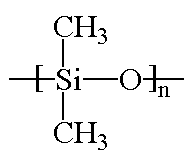
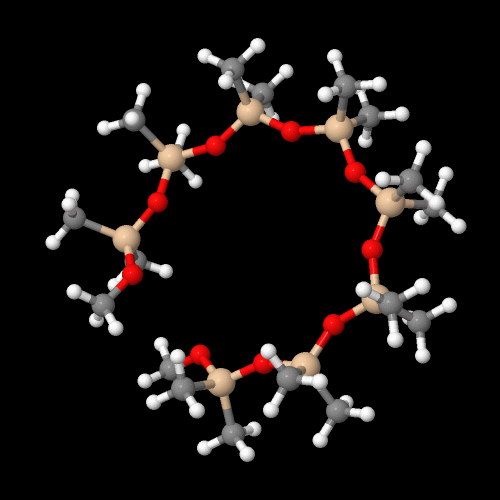
The model on the right above is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
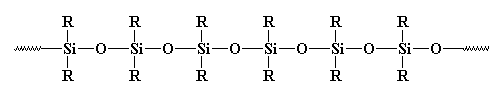
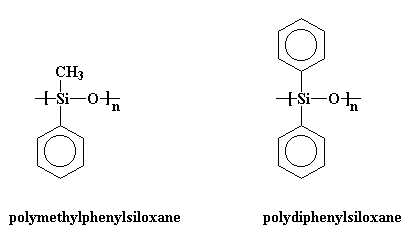
Silicones are inorganic polymers, that is, there are no carbon atoms in the backbone chain. The backbone is a chain of alternating silicon and oxygen atoms. Each silicone has two groups attached to it, and these can be any organic groups. The picture at the top of this page shows methyl groups attached to the silicon atoms. This polymer is called polydimethylsiloxane. It is the most common silicone. Want to see some others? Polymethylphenylsiloxane and polydiphenylsiloxane are also popular with the kids these days.
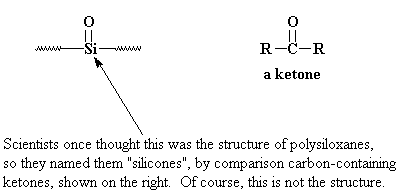
"Polysiloxane" is the proper name for silicones. But when they were discovered it was thought that they had "silicone" groups in the backbone chain. When the real structure was discovered, it was too late, and the name stuck.
Silicones make good elastomers because the backbone chain is very flexible. The bonds between a silicon atom and the two oxygen atoms attached to it are very flexible. The angle formed by these bonds can open and close like a scissors without much trouble. This makes the whole backbone chain flexible.
If you want to know how to make silicones, click here.
Polydimethylsiloxane does something really strange when you mix it with boric acid, or B(OH)3. The mixture is soft and pliable, you can mold it into any shape easily with your fingers. But it is also very bouncy. What's more, push it gently and it gives way, but hit it hard with a hammer and it cracks! Strangely, if you spread it over newspaper, and pull it away, it gets printed with a mirror image of the newspaper text. No industrial use was ever found for his wonder material, but tons of it have been sold as toy called Silly Putty.

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |