On most of these pages we've been talking about polymers whose backbone chains are made mostly of carbon atoms, if not entirely of carbon atoms. These we call organic polymers. But now we're going to leave convention behind and talk about some polymers that don't have any carbon atoms in the backbone chain. These are called, as if you couldn't guess, inorganic polymers. Here's a menu if the inorganic polymers on this page to help you navigate:
Silicones
You've seen inorganic polymers before -- if not on these pages, at least in
everyday life you've probably seen a silicone polymer
somewhere. Silicones are the most common inorganic polymers. They look
like this:
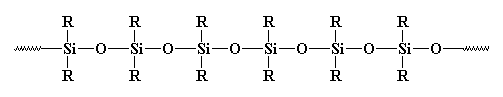
They really should be called polysiloxanes. The bond between silicon and oxygen is very strong, but very flexible. So silicones can stand high temperatures without decomposing, but they have very low glass transition temperatures. You've probably seen rubber or caulking made of silicones somewhere before.
Right?
Right. It took a long time to make it happen, but silicon atoms have
been made into long polymer chains. It was in the 1920's and 30's that
chemists began to figure out that organic polymers were made of long
carbon chains, but serious investigation of polysilanes wasn't
carried out until the late seventies.
Earlier, in 1949, about the same time
that novelist Kurt Vonnegut was working for the public relations
department at General Electric, C.A. Burkhard was working in G.E.'s
research and development department. He invented a polysilane called
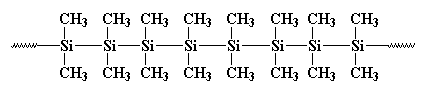
polydimethylsilane, but it wasn't much good for anything. It looked like
this:
It formed crystals that were so strong that
nothing could dissolve them. Burkhard tried to heat it, but it wouldn't
melt below 250oC, when it decomposed, without melting. That made
polydimethylsilane pretty much useless. He made it by reacting sodium metal with dichlorodimethylsilane like this:
This is important, because in the seventies, some scientists got the
notion that they were going to make small rings of silicon atoms. So
unwittingly did something similar to what Burkhard had done. They
reacted sodium metal with dichlorodimethyl silane, but they also added
some dichloromethylphenylsilane to the brew. And guess what happened!
I'll give you a hint: they didn't get the rings they wanted. What they
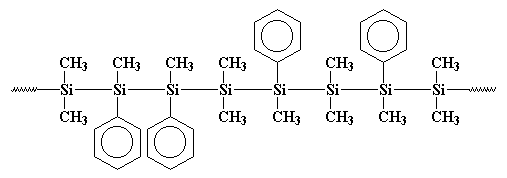
got was a copolymer, like this:
Maybe that polymer is more clearly drawn like this:
You see, those phenyl groups get in the way when the polymer tries to
crystallize, so it isn't as crystalline as polydimethylsilane. This
means it is soluble and can be processed and played with and studied.
So what are these good for? Polysilanes are interesting because they can
conduct electricity. Not as well as copper, mind you, but a lot better
than you'd expect for a polymer, and worth investigating. They're also very
heat resistant, almost up to 300 oC, but if you heat them a
lot higher you can make silicon carbide out of them, which is a useful
abrasive material.
Polystannanes are unique and nifty and wonderful and fabulous because
they are the only known polymers with backbones made entirely from metal
atoms. Like polysilanes, polygermanes and polystannanes are being
studied for use as electrical conductors.
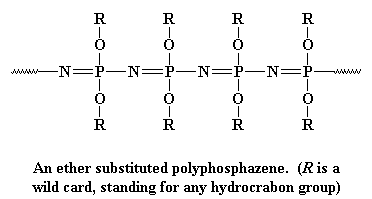
This backbone is very flexible, like the polysiloxane backbone chain, so
polyphosphazenes make good elastomers. They're
also very good electrical insulators. Polyphosphazenes are made in two
steps:
First we take phosphorus pentachloride and react it with ammonium
chloride to get a chlorinated polymer. Then we treat it with an alcohol
sodium salt, and that gives us an ether-substituted polyphosphazene.
Polysilanes
Let's take a look at the element silicon for a moment. You can see that
it's right beneath carbon in the periodic chart. As you may remember,
elements in the same column or group on the periodic chart often
have very similar properties. So, if carbon can form long polymer
chains, then silicon should be able to as well.
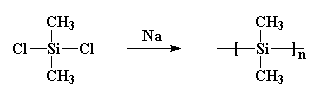

Polygermanes and Polystannanes
Okay, so if silicon can make long polymer chains, what about he other
elements in Group IV? Can you make polymers out of germanium? You'd
better believe you can! Not only can you make polymer chains out of
germanium, but you can even make a polymer chain out of tin atoms. These
polymers are called polygermanes and polystannanes, respectively.
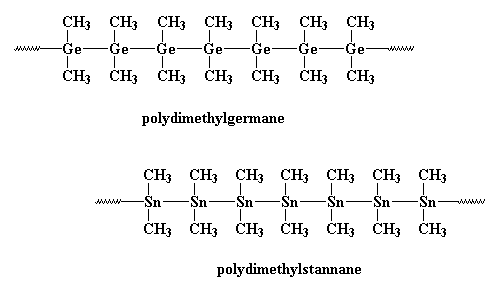
Polyphosphazenes
I hate to break it to you folks, but we're out of Group IV elements. So
the last inorganic polymer we're going to look at today is going to have
to be made of something else. And that something else is phosphorus and
nitrogen. Like the polysiloxanes, polyphosphazes are made of alternating
atoms, in this case, the chain is made up of alternating phosphorus and
nitrogen atoms, like this:
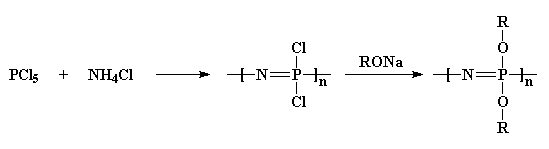

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |