
Miscible Polymer Blends
Keywords
amorphous,
copolymer,
entropy,
hydrogen bonding
Polymers Usually Don't Mix
Sometimes we want a material that has the some of the properties of one polymer, and some of the properties of another. Instead of going back into the lab and trying to synthesize a brand new polymer with all the properties we want, we try to mix two polymers together to form a blend that will hopefully have some properties of both in the right combination.
Sounds easy enough, but it turns out that blending two different kinds of
polymers can be really tricky business. You see, very seldom is it that
two different kinds of polymers will mix together. This doesn't seem to
make sense. Take a look at polyethylene and polypropylene here. Click on the model images below
if you want to play with the 3D model of each polymer.
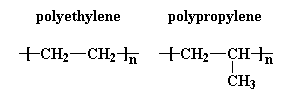
Would you believe that these two polymers don't mix? Why is that? What happened to the old "like dissolves like" rule that you learned in high school chemistry? These are both very non-polar hydrocarbon polymers. They should mix beautifully.
 But they don't. And yes, there is a reason why. It has to do with that old culprit entropy. Entropy is the name we scientists call disorder. This dog is named Entropy. Just say the word "Frisbee" around her and you'll get a good demonstration of what entropy is.
But they don't. And yes, there is a reason why. It has to do with that old culprit entropy. Entropy is the name we scientists call disorder. This dog is named Entropy. Just say the word "Frisbee" around her and you'll get a good demonstration of what entropy is.
This brings us to a little rule we call the second law of thermodynamics. The second law of thermodynamics says that when things change, they will change from a state of order to a state of disorder. Getting things to change in the other direction is very difficult. It's easy to mess up your room, but difficult to clean it up. It's easy to crash a car, but fixing it is much trickier. A change, in your room, in life, in polymers, is more likely to happen if that which is changing changes from a state of more order to a state of less order; that is, if it changes from a state of less entropy to more entropy.
So what does entropy have to do with polymer blends? This will take some explaining. Consider one type of polymer, in the amorphous state. When it's alone, by itself, all its chains are tangled up in each other randomly and chaotically. Entropy runs high in an amorphous polymer.
This presents a problem if you're trying to make polymer blends. You see, one of the biggest reasons two compounds will ever mix together is that they are more disordered mixed together than they are separate. So, mixing is favored by the second law of thermodynamics. But an amorphous polymer is so disordered as it is, that it really doesn't gain that much entropy when it's blended with another polymer. So, mixing is disfavored.
Making Polymers Mix
This presents a challenge to would-be polymer blenders. Without entropy to make polymers blend, how can we ever get two polymers to mix? To make that happen, we have to go back to go back to the first law of thermodynamics. Aha! Just like lawyers, we can use one law to get around another. The first law of thermodynamics says that when things change, they change from a state of more energy to a state of less energy. Think of it this way: it's easier to go to sleep than it is to get out of bed in the morning. Or if you'd like a physics example, a rock on top of a mountain will roll down to the bottom of the mountain more easily than a rock on the bottom will roll to the top. (I learned this the hard way when I was mountain climbing one summer and almost got killed by a falling boulder.)What, then, does this first law of thermodynamics have to do with blending polymers? This: in order to make two polymers mix, we have to make them have less energy when mixed than they would be separate. Let me use an example to illustrate. Two polymers that do actually mix are polystyrene and poly(phenylene oxide). Again, you can view the 3D models by clicking on the model images of the two polymers, right and left.
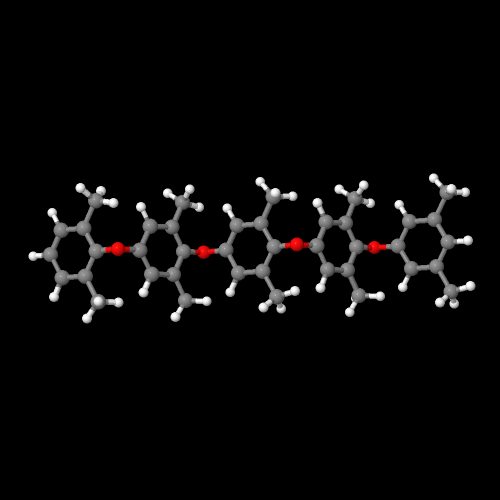
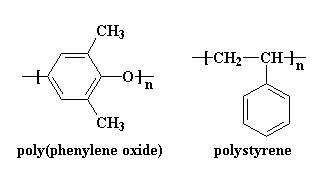
As you can see, both of these polymers have aromatic rings. As you may know, aromatic rings like to stack up like little hexagonal poker chips. For this reason, these two polymers like to associate with each other. So they blend very nicely.
There are a few other examples of polymer pairs which will blend. Here is a list of a few:
poly(ethylene terephthalate) with poly(butylene terephthalate)
poly(methyl methacrylate) with poly(vinylidene fluoride)
Copolymers
But most of the time, the two polymers you want to blend won't be miscible. So you have to play some tricks on them to make them mix. One is to use copolymers. Polystyrene doesn't blend with many polymers, but if we use a copolymer made from styrene and p-(hexafluoro-2-hydroxyisopropyl)styrene, blending is a lot easier.
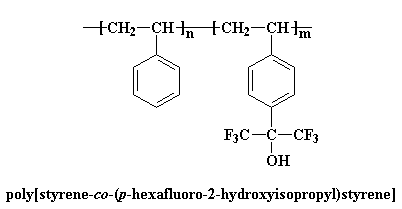
There's another way copolymers can be used to help polymers blend. Let's consider a random copolymer of styrene and acrylonitrile. This copolymer will blend with poly(methyl methacrylate) (PMMA). This is where it gets weird. PMMA won't blend with either polystyrene or polyacrylonitrile.
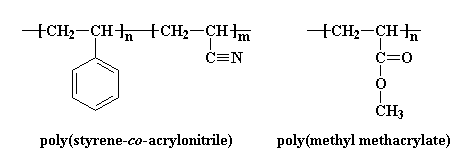
Making Your Own Blends
Blends are usually made in two ways. The first way is to dissolve two polymers in the same solvent, and then wait for the solvent to evaporate. When the solvent has all gone away, you'll be left with a blend at the bottom of your beaker, presuming your two polymers are miscible.While this method works fine in the laboratory, it could get expensive if you tried to do this industrially. Solvents aren't cheap, and if you're going to evaporate hundreds or thousands of gallons of them, you'll be paying a lot of money. Not to mention the effects on the environment of putting so much of your toxic solvents into the air, or the extra cost of recapturing all that solvent so it could be reused.
So for making blends in large amounts, you heat the two polymers together until you're above the glass transition temperatures of both polymers. At this point they will be nice and gooey, and you can mix them together like a cake mix. This is often done in machines such as extruders. When your material cools, you'll have a nice blend, again, presuming your two polymers are miscible.
Properties of Blends
So what are these blends like? How do they behave? In general, a miscible blend of two polymers is going to have properties somewhere between those of the two unblended polymers. Take for example the glass transition temperature, or Tg for short. If we take polymer A and blend it with polymer B, the Tg will depend on the ratio of polymer A to polymer B in the blend. You can see this in the graph below.
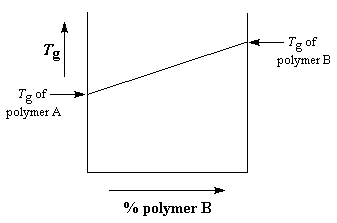
If polymer B has a higher Tg than polymer A, the Tg of the blend is going to increase as the relative amount of polymer B in the blend increases. The increase is generally linear, like you see in the graph. But the plot isn't perfectly linear. Sometimes if the two polymers bind more strongly to each other than to themselves, the Tg will be higher than expected, because the stronger binding lowers chain mobility. The plot will look like you see in the graph on the right below.
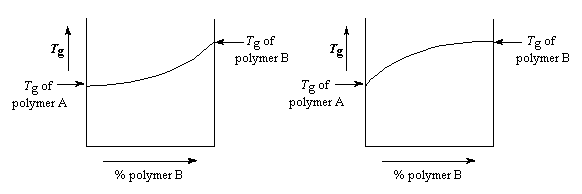
Of course, in most cases, the two polymers bind less strongly with each other than with themselves, so the Tgs of the blends are usually a little lower than expected. The Tg plot will look like the one you see above on the left.
We've been talking about Tgs up until now, but what holds for Tgs generally holds for other properties. Mechanical properties, resistance to chemicals, radiation, or heat; they all generally plot the same way as the Tg does with respect to the relative amounts of each polymer in the blend.
This makes altering the properties of your blend fairly simple. When you vary the amount of the two polymers, you vary the properties. This can be very useful. I'll use the example of poly(phenylene oxide), a.k.a. PPO, to illustrate. PPO is a very heat resistant polymer. This is wonderful. People need heat resistant materials. But it has some drawbacks. It's very hard to process. You see, it's too heat resistant. Amorphous polymers are usually processed by heating them above their Tgs so they get soft and gooey. But with a Tg of 210 oC, heating PPO enough to make it soft and gooey is not only difficult, but expensive.
Enter polystyrene. Remember, polystyrene and PPO blend nicely with each other. Since polystyrene has a Tg of only about 100 oC, blending polystyrene with PPO drops the Tg of the blend down to temperatures which make the blend much more processable than straight PPO.
Here's a nifty piece of information: NorylTM, the PPO/polystyrene blend that Sabic sells (formerly made by GE) , uses a special kind of polystyrene, called high-impact polystyrene, or HIPS for short. HIPS is really a mixture of polystyrene and polybutadiene. These two polymers don't blend. The rubbery polybutadiene separates from the polystyrene. But the little blobs of rubbery polybutadiene make HIPS, and NorylTM, a lot tougher. We call a mixture of two polymers like polystyrene and polybutadiene that phase separates an immiscible blend. Immiscible blends aren't really blends at all, because they phase-separate like water and chicken fat in a bowl of homemade chicken soup. But such phase-separated mixtures are also useful, mind you. If you want to read more about them go visit the Immiscible Polymer Blends Page.
To Blend or Not to Blend
A few polymer pairs mix. Most don't. But there are also polymer pairs that sometimes mix and sometimes don't. The variables that one can control to make them mix or not mix are usually temperature and composition. A lot of polymer pairs are only miscible when there is a lot more of one polymer than of the other. There will be a range of compositions for which the two polymers won't mix. For example, let's say we have two polymers, polymer A and polymer B. Let's also say they are miscible when we have less than 30% polymer B, that they are miscible when there is more than 70% polymer B. But between 30 and 70% polymer B, the blend phase-separates into two phases. Here's a graph for those who of you who like that sort of thing:
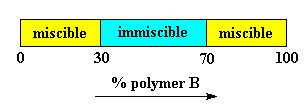
Interestingly, one phase will have 30% polymer B and the other will have 70% polymer B. There's a reason for this. If we look at a plot of free energy versus composition, we'll see that these two compositions are lower in energy than any other compositions.4 One note first: we chemists usually use the Greek letter f to represent the relative amount of one or the other component in a mixture of any kind, so we're going to use fB instead of "% B" from here on.
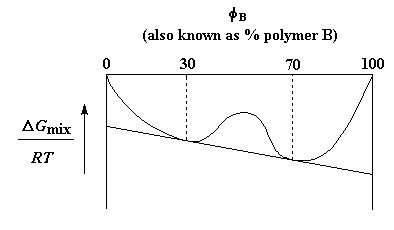
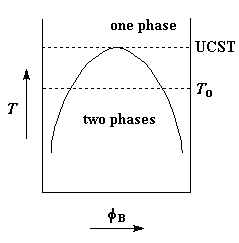 But the composition range over which the two polymers phase-separate isn't constant. It can change with temperature. For some polymer pairs that range gets smaller as temperature increases. Eventually, if you heat such a pair high enough, that range of immiscibility will become so small that it will disappear. The temperature at which this happens is called the upper critical solution temperature or UCST. The graph on the right shows this. The upside-down parabola is the boundary between those temperatures and compositions at which there is one phase, and those at which there is phase separation.
But the composition range over which the two polymers phase-separate isn't constant. It can change with temperature. For some polymer pairs that range gets smaller as temperature increases. Eventually, if you heat such a pair high enough, that range of immiscibility will become so small that it will disappear. The temperature at which this happens is called the upper critical solution temperature or UCST. The graph on the right shows this. The upside-down parabola is the boundary between those temperatures and compositions at which there is one phase, and those at which there is phase separation.
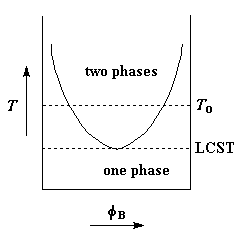
But sometimes the opposite happens. For some polymer pairs the range of immiscibility decreases with decreasing temperature. If one cools such a pair enough, eventually we'll reach a temperature at which the range gets so small that it disappears. This temperature is called the lower critical solution temperature or LCST. If one plots the range of immiscibility versus temperature, the plot looks like an inversion of the UCST plot, as you can see on the left.
Now for you thermodynamicists out there who are wondering what happens to our plot of free energy versus composition once we've crossed either a UCST or LCST and out polymer pair has become miscible in all compositions, we have a graph showing just that right here:
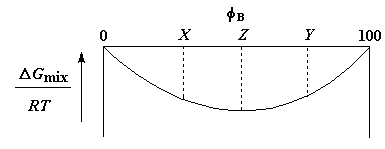
This plot takes some explaining. Imagine, if you will, a blend of polymers A and B, of composition Z. Now, imagine it phase-separating into two phases, one of composition X and the other of composition Y. As you can see, the two separate phases are both higher in free energy than the single phase at composition Z, so they are less stable than that single phase at composition Z. So, if the two separate phases were somehow generated they would spontaneously merge into one phase, whose composition is, you guessed it, Z.
References
1. Ikawa, T.; Abe, K.; Honda, K.; and Tsuchida, E.; J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 1505.2. Ting, E. P.; Pearce, E. M. and Kwei, T. K., J. Polym. Sci. Polym. Lett. Ed., 1980, 18, 201.
3. Pearce, E. M.; Kwei, T. K. and Min, B. Y., J. Macromol. Sci. Chem., 1984, 21, 1181.
4. Coleman, M. M., Graf, J. F. and Painter, P. et al., Specific Interactions and the Miscibility of Polymer Blends, Technomic, 1991, p.20.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |