| Goals | Nylon 6 Experimental |
| Objectives | Extension topics |
| Background | Safety considerations |
| Nomenclature | Notes |
| Theory | References |
| Nylon 6,10 Experimental | Self-test |
| Pre-lab Quiz |
For background information, visit these fun-filled Macrogalleria pages:
-
Nylons
Making Nylon 6,6
Making Nylon 6
Lab skills you will learn, if you don't already know them
Preparing of solutions of known concentrations
Using a Bunsen burner without burning yourself and without producing
deadly amounts of carbon monoxide
The proper technique for heating a test tube in a flame without shooting
the contents at your lab partner
Safe handling of pyrophobic materials such as NaH
Drawing fibers from a molten polymer without burning yourself
The Nylon Rope Trick, a neat visual demonstration
Concepts to be learned, if you don't already know them
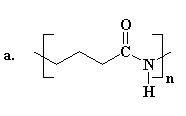
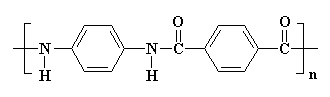
The structure of nylons
The different kinds of nylon and how they differ
The monomers from which nylons are produced
The reactions by which nylons are produced
Which reaction variables affect the properties of the product (e.g.,
molecular weight, tensile strength) and how.
What features of nylons make them good fibers
Commercial Importance
Nylons are some of the most important fibers produced commercially. If
you've ever slept in a tent or used a toothbrush, you've used nylon
fibers. But nylon can be more than just fibers. It's also used for
self-lubricating gears and bearings. Nylon-clay composites are used to
make under-hood automobile parts.
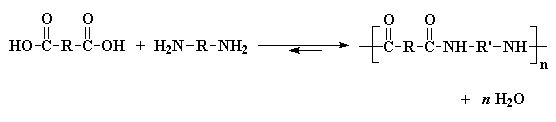
The two most important kinds of nylon are nylon 6,6 and nylon 6. These
two nylons have almost identical properties. Both were invented
in the late 1930s. Nylon 6,6 was discovered first. It was invented in the
United States by Wallace Carothers who was working for
DuPont.10 Not long after that Nylon 6 was invented in Germany
by Paul Schlack who was working for I.G. Farben.11
Physical Properties
You may ask yourself, "Why does nylon act as it does?" You may ask
yourself, "Why does nylon make such good fibers?" The answer to both is
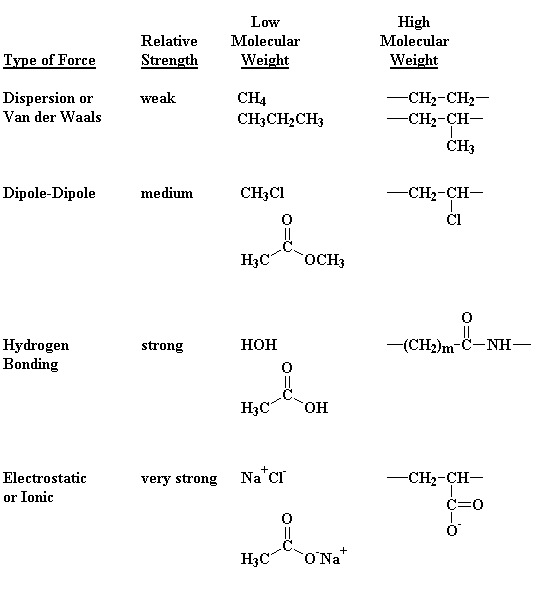
pretty simple: intermolecular forces. Just for review, Table 1 lists the
different kinds of intermolecular forces. When we're talking about
nylons, the most important intermolecular force is hydrogen bonding. The
nitrogen-bonded hydrogens of one nylon chain will hydrogen bond very
strongly with the carbonyl oxygens of another nylon chain. These hydrogen
bonds make crystals of nylon very strong, because they hold the nylon
chains together very tightly. Of course, these strong crystals make
strong fibers.
Unless it has been drawn into fibers, only about 20-30% of the nylon in a
given sample is crystalline when in solid form. The rest is in the
amorphous phase. But even though it's non-crystalline, the chains are
still bound strongly to each other by hydrogen bonds. This combination of
crystalline and strongly associated amorphous phases is what makes nylon
thermoplastics so tough. (This onlyapplies to nylons used as
thermoplastics, mind you. When drawn into fibers nylons become almost
entirely crystalline.
We all know that a lot of the nylon produced ends up as clothing. But it
also ends up as other everyday things like rope, tents, and toothbrush
bristles. Sometimes nylon is used to make the belts that reinforce tires.
Most passenger car tires have steel belts, but tires for aircraft, trucks
and off-road vehicles are often made of nylon. Under the hood of your
car you'll find nylon fibers reinforcing rubber belts,
too.
Goals
The primary goal of this exercise is to teach you the student the
fundamental laboratory skills necessary for making nylons. That's right,
YOU are going to be able to make nylon when we're through here.
In addition, this exercise seeks to teach you the fundamental concepts and
theories involved with nylon synthesis. We're going to do this so that
you know just WHY you're doing what you're doing, and not just following
instructions like little obedient sheep. Furthermore, by the time
you're done we hope that by having a firm grasp of the theory behind nylon
synthesis, and having mastered the hands-on skills involved, you'll know
how to alter the properties of your nylon by altering the appropriate
reaction conditions, plus be able to troubleshoot your reaction should
things go awry, all without having to go running to your TA to ask
what to do at every little step. You're here to learn how to be
independent, not codependent!
Objectives
The objectives to be reached in achieving the above stated goals fall into
two categories. First are the lab skills to be obtained, and second
are fundamental concepts to be learned. Let's list them:
Weighing out quantities of reactants
Background
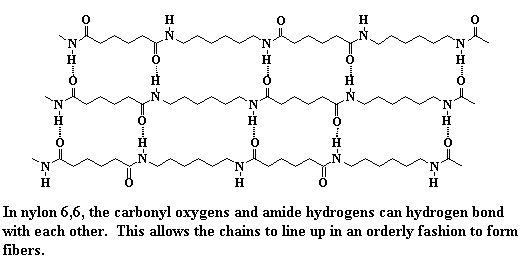
Table 1


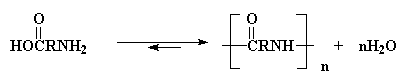
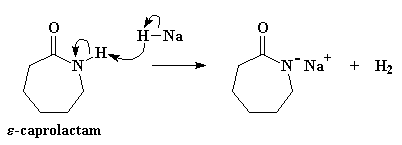
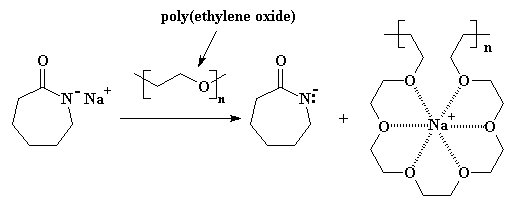
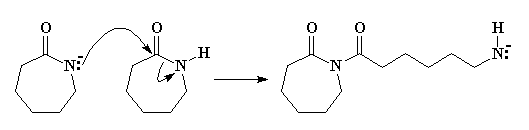
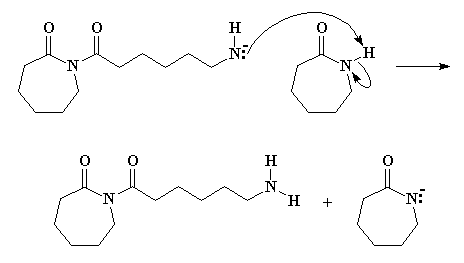
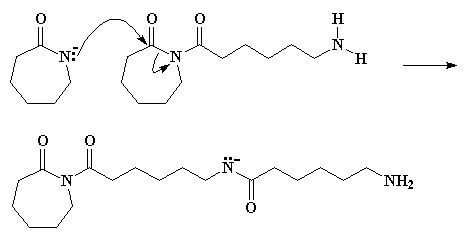
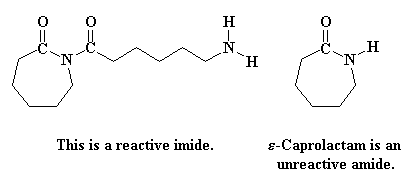
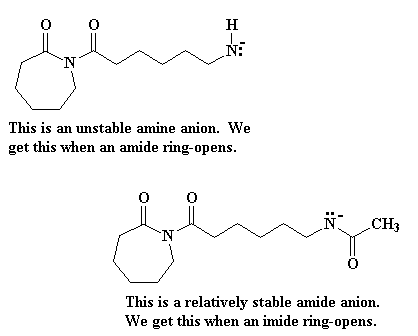
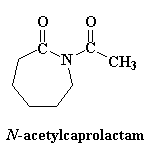
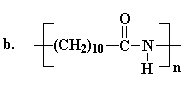
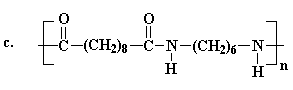
 Return to
USM Polymer Science Online Laboratory Directory
Return to
USM Polymer Science Online Laboratory Directory