

The image on the left is of the 3D model of nylon 6,6; on the right is nylon 6.
Just click on the image to view the model you can rotate and zoom in a new window.
Close it when you are ready to come back here.
For Nylon 6,6 at a glance, click here!
For Nylon 6 at a glance, click here!
Nylons are some of the most common polymers used as fibers. Nylon has excellent properties for use in fishing line and trimmer line, plus it's used for some "plastic" screws and push-in connectors. You'll find nylons in cars and in your home if you just look. Nylon is also found in clothing such as windbreakers, and (ahem...) lingerie, but also in other places, in the form of a thermoplastic.
Nylon's first real success came with it's use in women's stockings, in about 1940. They were a big hit, but they became hard to get, because the next year the United States entered World War II, and nylon was needed to make war materials, like parachutes and ropes. But before stockings or parachutes, the very first nylon product was a toothbrush with nylon bristles. At first, DuPont (the producer of commercial nylon 6,6) had a hard time figuring out to use it for. Once it caught on, though, markets opened up all over the place.
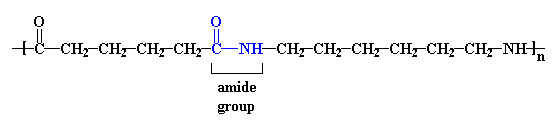
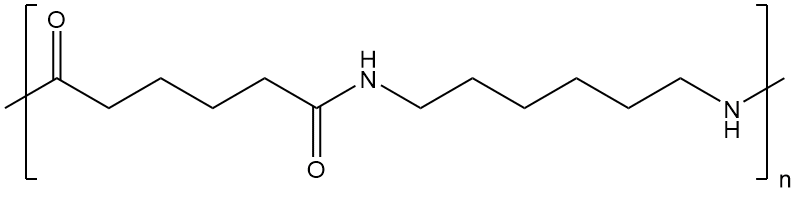
Nylons are also called polyamides, because of the characteristic
amide groups in the backbone chain. Proteins,
such as the silk nylon was made to replace, are also polyamides. These amide groups
are very polar, and can hydrogen bond with each other. Because
of this, and because the nylon backbone is so regular and symmetrical,
nylons
are often crystalline, and make very good fibers.
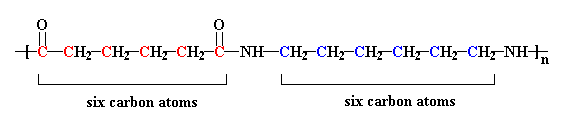
The nylon in the pictures on this page is called nylon 6,6, because each repeat unit of the polymer chain has two stretches of carbon atoms, each being six carbon atoms long. Other nylons can have different numbers of carbon atoms in these stretches.
Nylons can be made from diacid chlorides and diamines. Nylon 6,6 is made from the monomers adipoyl chloride and hexamethylene diamine.
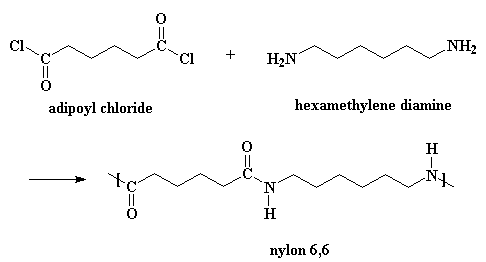
This is one way of making nylon 6,6 in the laboratory. But in a nylon plant, it's usually made by reacting adipic acid with hexamethylene diamine:
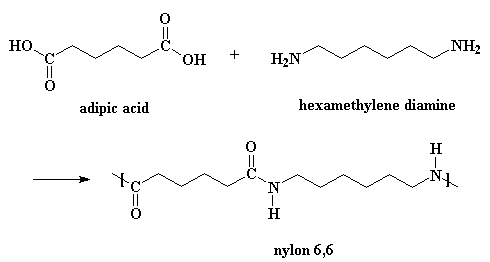
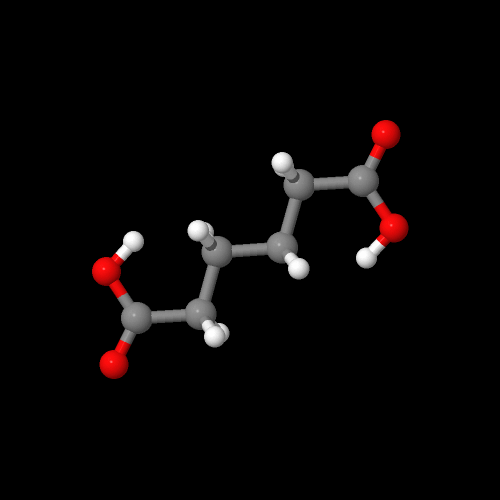
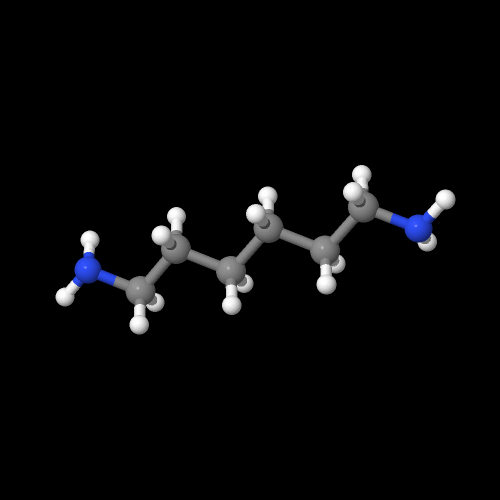
The model on the left is an image of the adipic acid model you can view by clicking here or you can just click on the image itself. The second image is of the diamine monomer. Either way, be sure to close the new windows that open up with the 3D model in it when you are ready to come back here.
If you want to know how this condensation polymerization works, click here.
Another kind of nylon is nylon 6. It's a lot like nylon 6,6 except that it only has one kind of carbon chain, which is six atoms long. It's made by a ring opening polymerization from the monomer caprolactam as shown below. Click on the image of nylon 6 on the right to open a new window with a 3D model of the polymer.
The image below is of the caprolactam pdb model you can view by clicking here or on the image.

Click here to find out more about this polymerization. Nylon 6 doesn't behave much differently from nylon 6,6 although its melting point is about 40 C lower. The main reason both were invented is because DuPont patented nylon 6,6 before anyone else could. Other companies had to invent nylon 6 in order to get in on the nylon business. Today, both are commercially made and sold into huge markets worldwide.
And now for a few unusual nylons that have limited or no industrial presence. The first two are A-B nylons like nylon 6, with repeat units consisting of a single chemical unit with one amine and one acid joined by methylene chains, or in the case of nylon 1, no methylenes at all. See the figures below for structures and overall syntheses for these two, each followed by tested procedures for making them.
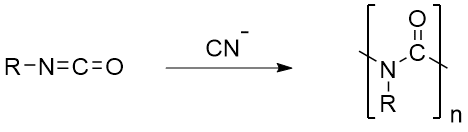
Click here to see the procedure and here to download a copy.

Click here to see the procedure and here to download a copy.
And for family of very different nylons with enormously better properties, see their own page at aramids to find out about polyamides with no methylenes and only aromatic groups connecting the amide moieties.
| Other polymers used as plastics include: | Other polymers used as fibers include: |
| Polypropylene | Polypropylene |
| Polyesters | Polyesters |
| Polystyrene | Polyethylene |
| Polycarbonate | Kevlar and Nomex |
| PVC | Polyacrylonitrile |
| Polyethylene | Cellulose |
| Poly(methyl methacrylate) | Polyurethanes |

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |

