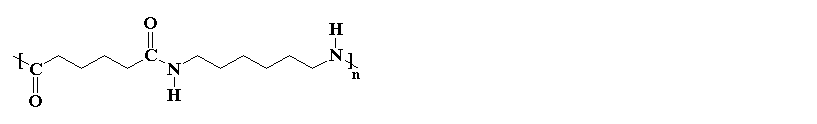
Nylons are one of the most common polymers used as a fiber. Nylon is found in clothing all the time today. In fact it is very similar to the natural polymer silk, which explains one of its earliest uses as a replacement for silk in women's stockings because silk was expensive and hard to get, especially in 1940 when this all happened. The new stockings were very popular, but soon the U.S. entered World War II, and all that nylon was needed for the war - to make parachutes and rope. Nowadays there's plenty of nylon to go around... and plenty of silk for that matter.
Nylon is also used in other places, such as in the form of a thermoplastic. Before stockings or parachutes, the very first nylon product was a toothbrush with nylon bristles.
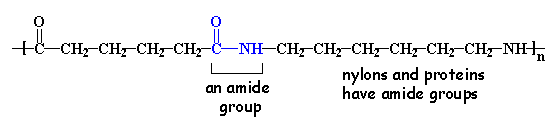
If you look at the nylon diagram above you'll see a chunk of atoms in blue. That chunk is called an amide group. Nylons are called polyamides, because of the amide groups in the backbone chain. Proteins, such as the silk nylon was made to replace, are also polyamides.
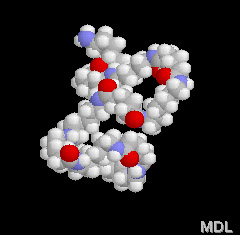 These amide groups are very polar and can hydrogen bond with each other. Because of this, and because the nylon backbone is so regular and symmetrical, nylons make great fibers because they are crystalline - which means the molecules all lock together in a regular pattern. It is easy to make out the nylon chain and it's pattern in the 3-D model on the right. Spin it around with your mouse and check it out!
These amide groups are very polar and can hydrogen bond with each other. Because of this, and because the nylon backbone is so regular and symmetrical, nylons make great fibers because they are crystalline - which means the molecules all lock together in a regular pattern. It is easy to make out the nylon chain and it's pattern in the 3-D model on the right. Spin it around with your mouse and check it out!
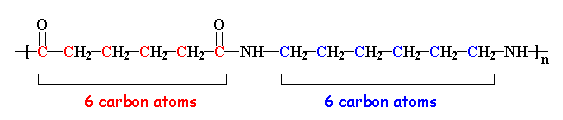
The nylon in the pictures on this page is called nylon 6,6, because each repeat unit of the polymer chain has two stretches of carbon atoms; each is six carbon atoms long. Other nylons can have different numbers of carbon atoms in these stretches.
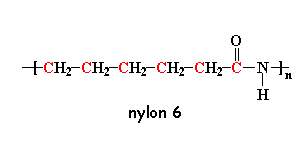
Another kind of nylon is nylon 6. It's a lot like nylon 6,6 except that it only has one kind of carbon chain, which is six atoms long.

NOW PLAYING |
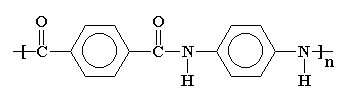
Aramids are a family of nylons, including Nomex® and Kevlar®. Kevlar® is used to make things like bulletproof vests and puncture resistant bicycle tires. I suppose one could even make bulletproof bicycle tires from Kevlar®.
Blends of Nomex® and Kevlar® are used to make fireproof clothing. Nomex® protects firefighters, race car drivers, and monster truck and tractor drivers from getting burned should their fire-breathing rigs breathe a little too much fire. Thanks to Nomex®, an important part of American culture can be practiced safely. (Polymers play another part in the monster truck show in the form of elastomers from which those giant tires are made.)
Aramids like Kevlar® are very crystalline polymers. Because of this they are used in the form of fibers. They form into even better fibers than other polyamides, like nylon 6,6.
|
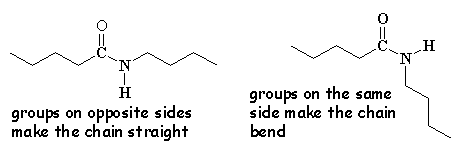
Check out this 3-D model of an aramid. It's not as twisty and bent as the model of nylon right below it. This is because every other pendant group in the aramids is on the opposite side of the chain. In regular nylons the order is more random, and often the groups will get on the same side of the chain and get in each other's way. This makes the chain bend and kink so it is not as straight. As you can see when you look at the two side by side, the aramids are more regular and spread out, so they interlock better to form very good strong fibers.
|
| Other polymers used as plastics include: | Other polymers used as fibers include: |
| Polypropylene | Polypropylene |
| Polyesters | Polyesters |
| Polystyrene | Polyethylene |
| Polycarbonate | Polyacrylonitrile |
| PVC | Cellulose |
| Polyethylene | Polyurethanes |
| Poly(methyl methacrylate) |

|
Return to Kinds of Polymers |

|
Return to Main Page |