Chronological Development of Polymeric Materials
SIGNIFICANCE OF THE HISTORICAL DEVELOPMENT OF
POLYMERS AND POLYMER SCIENCE
POLYMERS AND POLYMER SCIENCE
Want to try something interesting to get started? Take the “Why History is Important” quiz or the Polymer Dates quiz before you go any further! (Or you can wait till after you read the stuff below.)
Polymer Science- “Just a Babe in the Woods”
This has had unfortunate consequences, including the fact that polymer science has not been widely accepted as a legitimate field of study until recently. Even now, many “traditional discipline” scientists do not think polymer science is a “real” science perhaps because it doesn’t have it’s own language and culture (not true as we’ll find out). The result of this is that most individuals working in the field have not been trained in polymers, but in some closely related discipline like chemistry or chemical engineering. The basic ideas and unique concepts of polymer science are not consciously connected in their thinking but should be.
Traditional Science- Really “Grandfathered In”
For example, a B.S. scientist trained in chemistry has been taught mathematics, computer science, physics, synthesis, mechanisms and kinetics all within the context of “traditional chemistry”. Chemistry is the unifying theme or “umbrella” under which all these concepts are taught, although even in a chemistry department, the relationships are not clearly established: each sub-discipline of chemistry should be intimately related to all others in the chemist’s mind, but often aren’t. When a chemist approaches a problem, he should ideally see it as a problem in its totality and draw upon his entire background and training to ensure that all important aspects of that particular problem are addressed. What usually happens, however, is that he or she sees ALL problems as chemistry problems (not physics or engineering), and may focus even more narrowly on only one or two of the traditional areas. A better approach is to involve the entire field from organic to inorganic to physical and analytical. There’s an old saying that a man with a hammer sees everything as a nail.
Polymer Science- How it Should Be
Polymer science should also be taught with such a unifying approach, with “polymers” as the umbrella encompassing and pulling together chemistry, physics, mathematics and engineering. Individuals trained in polymers should be able to “think” in terms of polymer and materials problems, similar to how one learns to think in a foreign language. One learns words and grammar, then short sentences and paragraphs, and finally entire chapters and books with a unifying understanding that overlays and controls how one interacts with the world. Similarly, someone working in polymers must see relationships between synthesis of a polymer, its processing and history, and the final properties obtained. The figure below shows some of this connectivity for polymer science.

Ok, here’s a historical question you may know the answer to: who said “Those who fail to learn from history are doomed to repeat it.” If you answered Winston Churchill, bully for you! You’re wrong. It is actually a misquote of George Santayana (1863-1952), who, in his Reason in Common Sense, The Life of Reason, Vol.1, wrote “Those who cannot remember the past are condemned to repeat it.”

So now you know why history is important- so if someone asks you a trivia question, you can get it right! Well, actually there are lots of other reasons to learn history. In polymer science, the development of polymers, especially synthetic polymers, parallels our developing knowledge of chemistry over the last few hundred years. To the point: synthetic polymer science is a virtual sapling in the forest of human history (and prehistory, for that matter).
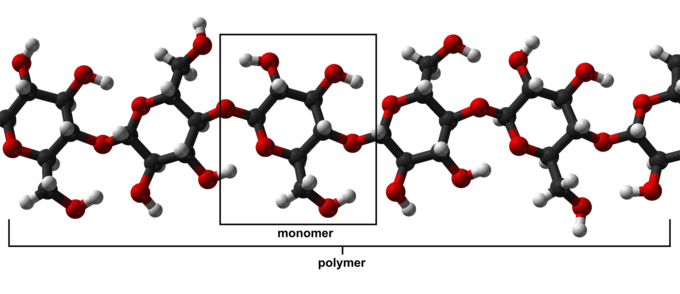
What do I mean by that prehistory comment? Just this: natural polymers have been around since life began on this planet. Think about wood, cotton, wool and silk. Now think a little deeper: every living creature is made up of many different kinds of polymers, from proteins and enzymes to RNA and DNA. So in a very real and living sense, you could not exist without polymers. Just to illustrate this point, take a look at the molecular structure of cellulose below. Lots of oxygens, carbons and hydrogens all joined together in with such specificity that even today we can’t easily make a synthetic analog. How did Mother Nature figure out how to make this incredibly complex polymer? And do so in living factories called “cotton plant” and trees? Think about all the wonderful uses of wood and where we’d be if we hadn’t had wood to make housing and tools from since time began…
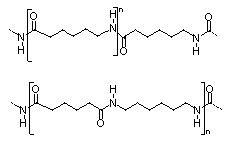
A key point here is this: we learn from nature. Not to make this trivial, since we learn lots of things from nature, but in the area of polymer science, it was our beginning understanding of natural polymers that led to so many synthetic ones. We looked at composition of proteins made from amino acids and applied those same general compositions to making synthetic polyamides we call nylons today. Crucial to the amazing properties of nylons (and proteins as well) are the hydrogen bonds that amide groups make along and between polymer chains. This particular hydrogen bond is between the N-H hydrogen and the C=O carbonyl oxygens (see nylon structure below).
So take a look below at just a few of the synthetic polymers we’ve made so far. You may not recognize all of them yet because they are often sold under trade names, such as “nylon” that I mentioned above. Kevlar, for example, is another polyamide, although its properties are incredibly improved by using aromatic (benzene-like) monomer units that natural polyamides don’t have. In short, we took our understanding of structure-property behavior of small molecules, applied it to synthetic polymers and came up with entirely new ways of making materials flexible, strong and incredibly tough. Does the term “bullet-proof vest” ring a bell?
When you’re done reading the material below, try the two quizzes linked at the top of the page. Don’t be afraid to guess- scores aren’t kept at this point. These quizzes help you understand what you know and don’t know. If you don’t do well, re-read the material and take them again. They’re fun and help you learn better.
| DATE | MATERIAL |
|---|---|
|
|
|
| <1800 | Natural fibers (cotton, flax, wool, silk, leather, wood, paper, starch, natural rubber) |
| 1839 | Vulcanization of rubber (Charles Goodyear) |
| 1868 | Celluloid (plasticized cellulose nitrate; Hyatt) |
| 1892 | Viscose rayon fibers (Cross, Bevan and Beadle) |
| 1907 | Phenol-formaldehyde resins (Bakelite; Baekeland) |
| 1927 | Poly(vinyl chloride) (PVC) wall covering |
| 1929 | Polysulfide synthetic elastomer (Thiokol; Patrick) |
| 1929 | Urea-formaldehyde resins |
| 1931 | Poly(methyl methacrylate) plastics (PMMA) |
| 1937 | Styrene-butadiene (Buna-S) and styrene-acrylonitrile (Buna-N) copolymer elastomers |
| 1938 | Melamime-formaldehyde resins |
| 1940 | Isobutylene-isoprene elastomer (butyl rubber; Sparks and Thomas) |
| 1943 | Fluorocarbon resins (Teflon, DuPont; Plunkett) |
| 1943 | Silicones and polysiloxanes |
| 1943 | Polyurethanes (Bayer) |
| 1947 | Epoxy resins |
| 1950 | Polyester fibers, poly(ethylene terephthalate) |
| 1956 | Polyoxymethylene (acetals) |
| 1957 | High-density (linear) polyethylene |
| 1957 | Polycarbonate from bisphenol-A |
| 1959 | cis-Polybutadiene and polyisoprene elastomers |
| 1960 | Ethylene-propylene copolymer elastomers (EPDM) |
| 1962 | Polyimide resins |
| 1964 | Poly(phenylene oxide), PPO GE |
| 1965 | Polysulfone |
| 1965 | Styrene-butadiene block copolymers (Shell Kraton SBR) |
| 1970 | Poly(butylene terephthalate) |
| >1971 | Poly(phenylene sulfide) |
| Isotactic Polypropylene | |
| Syndiotactic Polystyrene | |
| Nylon 6, polycaprolcatom | |
| Nylon 66, poly(hexamethylene adipamide) | |
| Poly(ether ether ketone), PEEK | |
| Poly(phenylene sulfide), PPS | |
| Poly(paraphenylene bisbenzoxazole) | |
| Kevlar and nomex (aramids) | |
| Poly(para-benzoate) (arylate) | |
| DIVEMA cyclopolymer | |
| Synthetic rubber (GRS) | |
| Taken in part from From R.B. Seymour and C.E. Carraher, Jr., “Polymer Chemistry,” Marcel Dekker, Inc., NY, 1981, and from the “Encyclopedia of Polymer Science,” John Wiley and Sons, New York | |
Now you should definitely try the Why History is Important quiz and the Polymer Dates quiz before you go any further! Be sure and hit the "back" arrow to come back here when you're done... And when you're ready, move on to definitions of some of the concepts related to the world of polymers: Polymer Definitions.