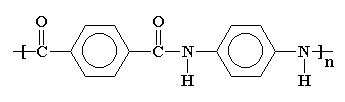
The model above is an image of the pdb model
you can view
by clicking here
or you can just click on the image itself.
Close the new window that opens up with the 3D model in it
when you are ready to come back here.
Aramids are a family of aromatic nylons that includes Nomex® and Kevlar.® Kevlar® is used to make things like bullet proof vests and puncture resistant bicycle tires. I suppose one could even make bullet proof bicycle tires from Kevlar® if one felt the need.
Blends of Nomex® and Kevlar� are used to make fireproof
clothing. Nomex� is what keeps the monster truck and tractor drivers from burning to death should their fire-breathing rigs breathe a little too much fire. Thanks to Nomex�, this important part of American culture can be practiced safely. Polymers play another part in the monster truck show in the form of elastomers from which those giant tires are made.

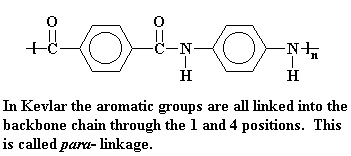
Nomex�, on the other hand, has meta-phenylene groups, that is, the amide groups are attached to the phenyl ring at the 1 and 3 positions. It's also more rigid than nylons and that rigidity is why it doesn't melt or burn in flames.
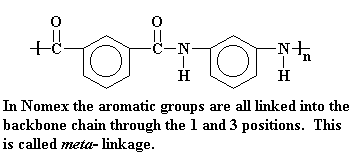
The syntheses of these aramid polymers requires very reactive monomers. Specifically, an aromatic diacid chloride is reacted with an aromatic diamine. The commercial polymers sold by DuPont use para with para monomers and meta with meta. You can view the monomers here.
Kevlar� is a very crystalline polymer. It took a long time to figure out how to make anything useful out of Kevlar� because it wouldn't dissolve in anything. Processing it in a solution was out. And it wouldn't melt below a right toasty 500 oC, so melting it down was out, too, which eliminated melt extrusion to make fibers. Then a scientist named Stephanie Kwolek came up with a brilliant plan. Click here to find out what it was.
Aramids are mostly used in the form of fibers. They form into even better fibers than non-aromatic polyamides, like nylon 6,6. That's mainly because the stiff backbone makes the chains all line up parallel to each other. This gives aramid fibers enormous tensile strength plus high melting points, both good things even if expensive to make.
Why? Why?
Ok, since it seems everyone just has to know, I'll tell you. It has to do with a little quirky thing that amides do. They have the ability to adopt two different shapes, or conformations. You can see this in the picture of a low molecular weight amide. The two pictures are the same compound, in two different conformations. The one on the left is called the trans conformation, and the one on the right is the cis- conformation.
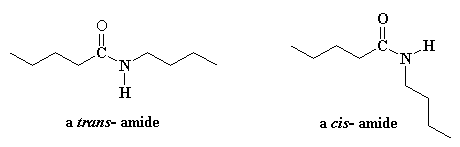
In Latin, trans means "on the other side". So when the hydrocarbon groups of the amide are on opposite sides of the amide bond, the bond between the carbonyl oxygen and the amide nitrogen, it's called a trans-amide. The methylene chains are co-linear to each other. That is, they line up perfectly. In a related definition, cis in Latin means "on the same side." When both hydrocarbon groups are on the same side of the amide bond, we call it a cis-amide. This bond makes a "kink" in the chain so that it can't line up easily in a parallel direction alongside other nylon chains.
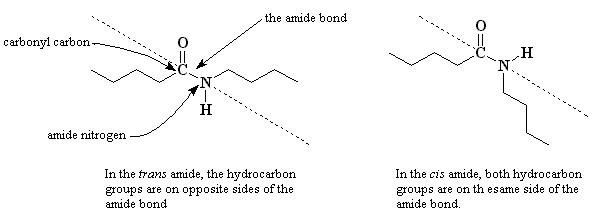
The same amide molecule can twist back and forth between the cis- and trans- conformations, given a little bit of thermal energy. Actually, we can see both types of bonds in nylons using carbon NMR. But this requires the polymers be dissolved in a good NMR solvent like CDCl3. A neat way to do this is to add a third or so by volume of trifluoroethanol (TFE). The combination of these two solvents will dissolve nylons and make low viscosity solutions that are perfect for NMR analysis.
The same cis- and trans-conformations exist in all polyamides, to a greater or lesser degree. When all the amide groups in a polyamide (like nylon 6,6 for example) are in the trans conformation, the polymer is fully stretched out in a straight line. This is exactly what we want for fibers, because long, straight, fully extended chains pack more perfectly into the crystalline form that makes up the fiber. But sadly, there's always at least some amide linkages in the cis-conformation. So nylon 6,6 chains never become fully extended, never all line up in the same direction, and never achieve their full strength potential.
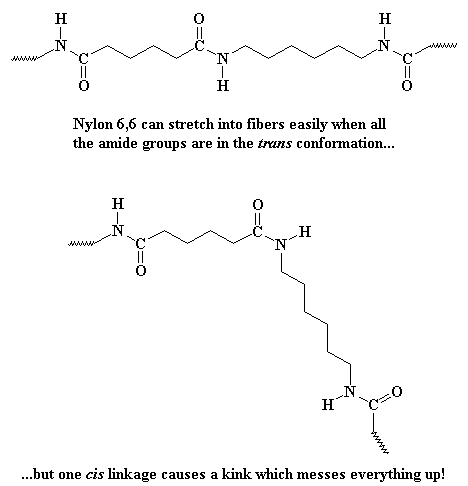
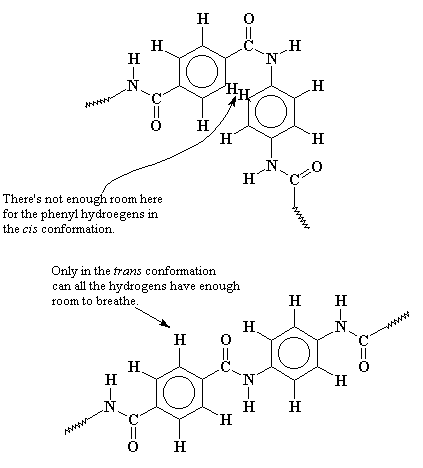
But Kevlar� is different. When it tries to twist into the cis-conformation, the hydrogens on the big aromatic groups get in the way! The cis conformation puts the hydrogens just a little closer to each other than they want to be. So Kevlar� stays nearly fully in the trans- conformation. So Kevlar� can fully extend to form beautiful fibers.
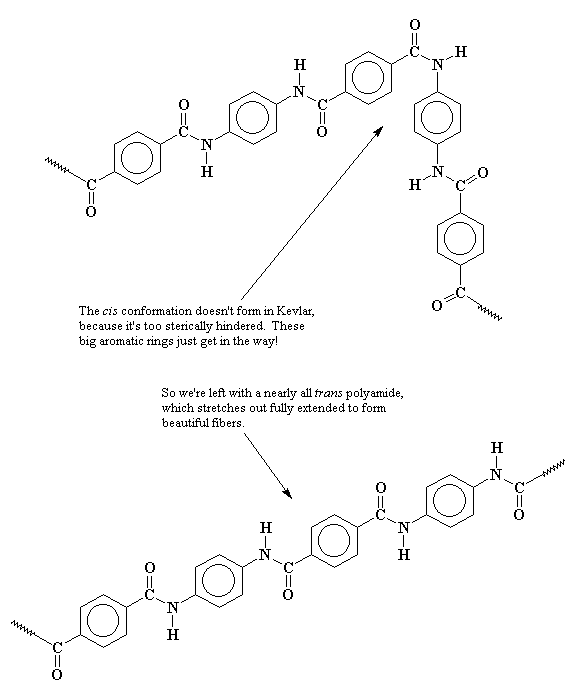
Now it may help to look at a close-up picture of this. Look at the picture below and you can see that when Kevlar� tries to form the cis-conformation, there's not enough room for the phenyl hydrogens. So only the trans-conformation is usually found.
But there's another polymer that stretches out even better called ultra-high molecular weight polyethylene. It even replaced Kevlar� for making bullet-proof vests!
But back to Kevlar�...
The phenyl rings of adjacent chains stack on top of each other very easily and neatly, which makes the polymer even more crystalline, and the fibers even stronger. That's because the phenyl rings are pretty "flat" and are co-planar with the amide bonds which are also pretty flat. Think of tongue depressors all lined up in a box, packed tight next to each other because they're all flat and long.
Detailed Synthetic Procedures
So recent advances in methods allow the simple, straightforward (and safe) syntheses of aramids. Let's look at that for the A-B aramid, poly(p-benzamide): click here to view the procedure and here to download a copy.
And now for the Kevlar-like AA-BB polymer made by click this procedure or download a copy by clicking here.
Other polymers that are used as fibers include:

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
